Detection of rearrangement of anaplastic lymphoma kinase (ALK) and mutation of epidermal growth factor receptor (EGFR) in primary pulmonary lymphoepithelioma-like carcinoma
Introduction
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) was first reported by Bégin et al. (1) in 1987. In the 2015 World Health Organization (WHO) Classification of Lung Tumors (2), LELC was moved to a group of “other and unclassified carcinomas” from large cell carcinoma. Histopathologically it is similar to nasopharyngeal lymphoepithelioma (3), which is a type of undifferentiated carcinoma with predominant lymphocytic infiltration most commonly occurred in Southern China, and had close relationship with Epstein-Barr virus (EBV) infection (4). Over the past 28 years since it was first reported, less than 300 cases have been reported in the literature. The state-of-art treatment for early stage disease is complete resection; whereas multimodality treatment strategy (surgery, chemotherapy, radiotherapy) applied in locally advanced disease, and palliative chemotherapy was used for metastatic disease. Compared with other types of non-small cell lung cancer (NSCLC), patients with pulmonary LELC had significantly better prognosis (5). However, due to the rarity of pulmonary LELC, treatment for advanced pulmonary LELC is still controversial.
Epidermal growth factor receptor (EGFR) is a protein that helps regulate cell growth. Abnormalities in the EGFR gene can lead to the development of NSCLC (6). EGFR mutations are more often found in the adenocarcinoma. In the western country, about 15% NSCLC patients have an EGFR mutation; however, in Asian countries this number is as high as 40-50% (7). Recently, studies have found that echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion gene plays an important role in the pathogenesis of lung cancers, and ALK fusion gene can be found in nearly 5-8% of NSCLC patients (8). Patients with NSCLC who have sensitizing EGFR mutation responded better to EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib or erlotinib (9), and patients with the ALK rearrangement were sensitive to ALK TKIs such as crizotinib (10). Testing for EGFR gene mutations and ALK rearrangements are routine for NSCLC patients in clinical practice now (11,12). However, only few genotype studies have been done in pulmonary LELC, and till now no targeted therapy has been shown effective in the treatment of these patients. Tam et al. (13) observed that EGFR mutations were uncommon in LELC (1 of 11 patients were positive) and Chang et al. (14) reported that 17.4% of patients with lung LELCs harbored EGFR mutations. As we previously reported (5), we analyzed EGFR mutations in 11 patients with LELC of the lung, but all patients were wild-type. All these data suggested that EGFR target therapy may not be an encouraging treatment for patients with advanced LELC of the lung (14). The EML4-ALK expression profile in pulmonary LELC was only reported in 11 patients by Wong et al. (15) but no patients had observed this fusion gene. Thus, in this relatively large cohort of patients with pulmonary LELC, we investigated the prevalence of EGFR mutation and ALK rearrangement, trying to explore the future targeted therapy in primary pulmonary LELC.
Materials and methods
Ethics, consent and permissions
Approval to review, analyze, and publish the data in this study was given by the Sun Yat-sen University Cancer Center Research Ethics Board. Written informed consent for the collection of medical information was obtained from all patients at their first visit.
Patients
We retrospectively investigated a cohort of 42 patients who were diagnosed with primary pulmonary LELC and treated in Sun Yat-sen University Cancer Center from January 2008 to April 2014. Pulmonary LELC was diagnosed according to criteria described by the WHO (2). We excluded undifferentiated carcinomas without lymphoid infiltrates and EBV-encoded RNAs (EBERs) staining in our study. All patients underwent endoscopic examination of the nasopharynx or PET-CT scan to rule out metastatic LELC from the nasopharynx.
We collected the clinical data from patients’ medical records. We focused on patients’ gender, age, symptoms, smoking status, tumor size, staging and treatments. Pathologic or clinical staging was performed according to the American Joint Committee on Cancer (AJCC) staging system (the 2007 TNM classification of malignant tumors) (16). Tumor assessment was based on Response Evaluation Criteria in Solid Tumors after at least two cycles of chemotherapy (17).
Immunohistochemistry staining
Immunohistochemical staining with CK5/6 (DAKO), CK7 (DAKO), P63 (Santa Cruz), and TTF-1 (DAKO) was carried out and evaluated according to the manufacturer’s instructions.
In situ hybridization (ISH) of Epstein-Barr virus-encoded RNAs (EBERs)
EBERs were detected using the EBV Probe In Situ Hybridization Kit (DIG-AP, A300K.9901, PanPath Company, Amsterdam, Netherlands) as described in previous reports (18). Briefly, the process included the following steps: (I) deparaffinization and dehydration of the paraffin sections using xylene and a series of graded ethanol; (II) pretreatment with 0.4% pepsin for 10 minutes; (III) hybridization with digoxigenin-conjugated EBV (EBERs) probe at 37 °C for three hours; (IV) signal detection using peroxidase-conjugated anti-digoxigenin antibody and 3,3’-diaminobenzidine (DAB); and (V) counterstaining the sections with hematoxylin solution. The positive signals were brownish-yellow and localized within the nuclei.
Mutational analysis of EGFR
Mutational analysis of the EGFR (exons 18 through 21) was carried out using TaqMan real-time polymerase chain reaction (PCR) as described in previous studies. Briefly, tissue sections of 10-µM thickness microdissected from formalin-fixed paraffin-embedded surgically resected tumor specimens were examined by microscopy after hematoxylin and eosin staining, and only tissue samples with greater than 80% tumor content were selected for the study. To obtain genomic DNA, the QIAamp DNA FFPE tissue kit (Qiagen, Hilden, Germany) was used according to the manufacturer’s instructions. The EGFR mutations were analyzed using a Real Time PCR Detection Kit for the Analysis of EGFR Gene Mutations (GP Medical Technologies, Beijing, China), to detect two specific in-frame deletion mutations in exon 19 (A: E746-A750del B: L747-P753ins S del) and two point mutations in exon 21 (C: L858R D: L861Q) of the EGFR gene. The TaqMan PCR and genotyping analysis were performed on ABI7500 Real Time PCR System (Applied Biosystems, Life Technologies Corporation, Carlsbad, CA). Data were analyzed with SDS2.0.software (Applied Biosystems) according to the manufacturer’s instructions.
ALK rearrangement determination
Determination of ALK rearrangement status was done by FISH using Vysis ALK Break Apart FISH Probe Kit (Abbott Molecular, Inc.) according to the manufacturer’s instructions. This commercial kit includes orange and green-colored break apart probes which flank, respectively, the 5 and 3 sides of the translocation breakpoint within the ALK gene. If ALK is not rearranged, the two probes overlap in a fused or yellow signal; ALK rearrangement is characterized by spatial separation of the green and red probes or by an isolated orange signal. Criteria that need to be met for a break apart FISH assay to be considered positive for ALK rearrangement include: at least 15% of the cells counted to harbor signals of translocation; separation of the green and red signals by at least two signal diameters; and at least 50 cells counted (19).
Results
The clinicopathologic characteristics of 42 patients with pulmonary LELC are presented in Table 1. The female to male ratio was about 1:1, and the median age at diagnosis was 51 years (range, 29-67 years). Only 13 (31.0%) patients were smokers. Seventeen patients (40.5%) had no symptoms at the time of diagnosis, and were found to have LELC by regular checkups. Other patients had mild cough (10 patients), chest pain (7 patients), or bloody sputum (8 patients). Twenty-seven of 42 patients had stage I-IIIA (64.3%), and only 15/42 patients (35.7%) were in stage IIIB or IV.
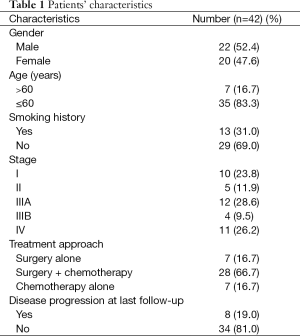
Full table
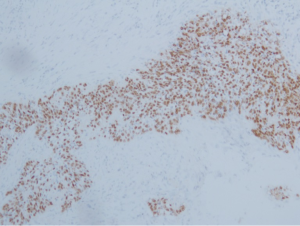
All specimens were from primary tumors. Pulmonary LELC was characterized by undifferentiated carcinoma cells with ill-defined cytoplasmic borders arranged in syncytial sheets and nests. The tumor cell nuclei were round, oval or elongated, with mildly irregular nuclear borders, vesicular chromatin and distinct nucleoli. The stromal tissue septa contained large numbers of reactive lymphoplasmacytic cells and other inflammatory cells (Figure 1A and B). By immunohistochemical staining, all patients demonstrated positive expression of CK5/6 (Figure 1C) and P63 (Figure 1D), but almost all patients were negative for TTF-1(34/34, 100%) or CK7 (34/35, 97.1%). ISH for EBERs showed positive signals in all 42 patients (Figure 2). Of 42 patients tested, only one patient (2.4%) harbored EGFR L858R mutation (Figure 3A) while others were all EGFR wild-type. None of the 42 patients had ALK rearrangement (Figure 3B).


As was shown in Table 1, multimodality treatment was applied in 66.7% of patients. Most chemotherapy regimens were platinum-based (cisplatin or carboplatin combined with pemetrexed, gemcitabine, or docetaxel). Of note, EGFR TKI gefitinib was applied in that patient with EGFR L858R mutation, but no objective response was observed and the PFS was only 1 month. None of those 42 patients received ALK-targeted therapy crizotinib due to lack of ALK rearrangement. At a median follow-up time of 12 months (range, 1-67 months), 8 patients had disease progression at a median of 10 months (range, 1-40 months). All patients were alive at the time of data analysis.
Discussion
Pulmonary LELC is a rare histological type of lung cancer and the incidence in previous reports is approximately 1% of all lung malignancies (14). Its clinical and pathological features are different from other histological types of NSCLC. Smoking was considered to be a major cause of lung cancer. However, only 31.0% of LELC patients in this study had a smoking history, which suggested that smoking might not play an important role in the tumor genesis of LELC (5). Nearly half of our patients were asymptomatic when they were diagnosed with pulmonary LELC, whereas others present with symptoms similar to those observed in other lung cancers, such as cough, chest pain, bloody sputum, etc.
In this study, all patients had positive expression of CK5/6 and P63, while almost without expression of TTF-1, indicating the nature of pulmonary LELC is more likely to be squamous cell carcinoma rather than adenocarcinoma. As was mentioned above, LELC is histopathologically identical to the nasopharyngeal lymphoepithelioma, suggesting chemotherapy drugs which were sensitive to nasopharyngeal carcinoma (NPC) such as paclitaxel/docetaxel, 5-fluorouracil, capecitabine and cisplatin may be effective in LELC. In our cohort, 12 patients received paclitaxel/docetaxel-based therapy (alone or combined with cisplatin), and 4/12 (33.3%) patients got PR, and 5/12 (41.7%) got SD. Ho et al. (20) reported their experience in five patients with advanced or metastatic pulmonary LELC treated with single agent capecitabine as salvage chemotherapy. Disease control was obtained in three of five patients, especially with exceptionally durable stable disease (14.8 months) in one patient, suggesting the potential clinical activity of capecitabine in pulmonary LELC. Recently, more and more studies have found that gemcitabine can induce lytic EBV infection in B-cell lymphomas, and addition of ganciclovir to gemcitabine can enhance the therapeutic efficacy for EBV-driven tumors (21). As previously reported, all Chinese pulmonary LELC patients had positive EBERs, confirming the consistent association of EBV in Chinese patients with pulmonary LELC, and suggesting a possible etiologic role of EBV (4). Thus, combination of ganciclovir and gemcitabine-based chemotherapy could be further investigated in the treatment of pulmonary LELC.
In patients with advanced NSCLC, progress has been made in identifying patients whose disease is caused by specific genetic alterations. This has enabled the development of therapies targeted against different oncogenic drivers, in particular in the EGFR mutations and ALK rearrangements. To our knowledge, this study is the largest cohort to date reporting prevalence of EGFR mutation and ALK rearrangements in primary pulmonary LELC.
Mutation in EGFR tyrosine kinase domain encoded by exons 18-21 is involved in both pathogenesis and progression of lung cancer (6). EGFR TKIs (erlotinib, gefitinib) are now the standard of care in patients with advanced/metastatic NSCLC harboring sensitizing EGFR mutations. An earlier study on the detecting EGFR mutations among different histological subtypes of NSCLC demonstrated a low incidence (1/11, 9.1%) of EGFR mutations in pulmonary LELC (13). Chang et al. (14) found that EGFR mutations were identified in eight out of 46 pulmonary LELC patients (17.4%), among which there were three patients with mutations in exon 21, two with mutations in exon 20, one with mutation in exon 19, and one with mutation in exon 18. Studies had shown that in-frame deletion in exon 19 and L858R mutation in exon 21 are predominant mutations in lung cancer (22,23). In our cohort, among 42 detected patients, only one (2.4%) patient harbored EGFR L858R mutation in exon 21, but responded poorly to EGFR TKIs treatment and PFS was only one month. In all, these results indicated that EGFR mutation is uncommon and maybe not an oncogenic driver gene for primary pulmonary LELC which should be further investigated (24).
Rearrangement or fusion of the ALK gene, which ultimately gave rise to the oncogenic ALK fusion kinase, was identified as a driver oncogene of lung cancer in 2007 (25). The first ALK fusion identified in NSCLC was EML4-ALK, although other fusion partners for ALK in NSCLC have since been discovered (26), along with several EML4 variants (27). ALK rearrangement was also a known oncogenic driver in tumors such as anaplastic large cell lymphoma and inflammatory myofibroblastic tumor (28). It is estimated that ALK rearrangements occur in around 5-8% of NSCLC cases depending on the population selection (8). The ALK inhibitor crizotinib and ceritinib were approved for advanced or metastatic NSCLC with ALK-rearrangement (29,30). In our study, we used ALK break apart FISH probe to detect ALK rearrangement. One of the major advantages of the break apart FISH assay was that this method does not rely on knowledge of all ALK fusion partners, an important benefit since some remain to be discovered (19). A further advantage of the technique was highly sensitive and specific. However, in this cohort, none of 42 patients with pulmonary LELC had ALK rearrangement, which is consistent with previously report (15). Thus, we concluded that ALK rearrangement was possibly not a driver gene and ALK-targeted therapy might not be favorable for pulmonary LELC.
In conclusion, primary pulmonary LELC is a special histological subtype of lung cancer with rare EGFR mutation and lack of ALK rearrangement. Whether EGFR-targeted therapy is effective in pulmonary LELC patients harbored with EGFR mutations should be further studied. Conventional cytotoxic chemotherapy is still a backbone treatment in advanced stage primary pulmonary LELC. Future efforts should be made to explore other oncogenic driver gene to guide targeted therapy in this rare disease to determine the optimal treatment.
Acknowledgements
The authors thank the pathologists from the Department of Pathology at the Sun Yat-sen University Cancer Center for their cooperation.
Funding: This work was supported in part by Department of Health of Guangdong Province (B2009089), Guangdong Science and Technology Department (2012B031800116), the Science and Technology Planning Project of Guangdong Province (2010B31500010).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclosure: Presented in part at the 16th IASLC World Conference on Lung Cancer (WCLC 2015), September, 2015, Denver, USA.
References
- Bégin LR, Eskandari J, Joncas J, et al. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol 1987;36:280-3. [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [PubMed]
- Han AJ, Xiong M, Zong YS. Association of Epstein-Barr virus with lymphoepithelioma-like carcinoma of the lung in southern China. Am J Clin Pathol 2000;114:220-6. [PubMed]
- Ngan RK, Yip TT, Cheng WW, et al. Clinical role of circulating Epstein-Barr virus DNA as a tumor marker in lymphoepithelioma-like carcinoma of the lung. Ann N Y Acad Sci 2004;1022:263-70. [PubMed]
- Liang Y, Wang L, Zhu Y, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer 2012;118:4748-58. [PubMed]
- Italiano A, Saint-Paul MC, Caroli-Bosc FX, et al. Epidermal growth factor receptor (EGFR) status in primary colorectal tumors correlates with EGFR expression in related metastatic sites: biological and clinical implications. Ann Oncol 2005;16:1503-7. [PubMed]
- Johnson BE, Jänne PA. Epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Cancer Res 2005;65:7525-9. [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [PubMed]
- Tam IY, Chung LP, Suen WS, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res 2006;12:1647-53. [PubMed]
- Chang YL, Wu CT, Shih JY, et al. Unique p53 and epidermal growth factor receptor gene mutation status in 46 pulmonary lymphoepithelioma-like carcinomas. Cancer Sci 2011;102:282-7. [PubMed]
- Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. [PubMed]
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [PubMed]
- Huang YH, Wu QL, Zong YS, et al. Nasopharyngeal extranodal NK/T-cell lymphoma, nasal type: retrospective study of 18 consecutive cases in Guangzhou, China. Int J Surg Pathol 2011;19:51-61. [PubMed]
- Camidge DR, Kono SA, Flacco A, et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res 2010;16:5581-90. [PubMed]
- Ho JC, Lam DC, Wong MK, et al. Capecitabine as salvage treatment for lymphoepithelioma-like carcinoma of lung. J Thorac Oncol 2009;4:1174-7. [PubMed]
- Feng WH, Hong G, Delecluse HJ, et al. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J Virol 2004;78:1893-902. [PubMed]
- Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839-44. [PubMed]
- Takano T, Ohe Y, Sakamoto H, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol 2005;23:6829-37. [PubMed]
- Liu Q, Ma G, Yang H, et al. Lack of epidermal growth factor receptor gene mutations in exons 19 and 21 in primary lymphoepithelioma-like carcinoma of the lung. Thoracic Cancer 2014;5:63-67.
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2008;14:6618-24. [PubMed]
- Takeuchi K, Choi YL, Togashi Y, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res 2009;15:3143-9. [PubMed]
- Pulford K, Morris SW, Turturro F. Anaplastic lymphoma kinase proteins in growth control and cancer. J Cell Physiol 2004;199:330-58. [PubMed]
- Ou SH. Crizotinib: a novel and first-in-class multitargeted tyrosine kinase inhibitor for the treatment of anaplastic lymphoma kinase rearranged non-small cell lung cancer and beyond. Drug Des Devel Ther 2011;5:471-85. [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [PubMed]


