Lower airway inflammation and hyperresponsiveness in non-asthmatic patients with non-allergic rhinitis
Introduction
Rhinitis is very common worldwide. Approximately two thirds of cases have been diagnosed as allergic rhinitis (AR) and the remaining as non-allergic rhinitis (NAR) (1-4). Asthma has also been a common respiratory disease, with more than 30 million cases globally (5). AR has been associated with the development of asthma (6), possibly because of their similar underlying inflammatory responses. The link between AR and asthma, in terms of underlying causes, pathophysiology and intervention approaches, has been increasingly recognized (7). Approximately 80% of patients with asthma also have rhinitis (1). The integrity of airway inflammation has been elaborated in the guideline, Allergic Rhinitis and its Impact on Asthma (1,8,9). This guideline recommended the combination therapy for upper and lower airways in patients with AR and asthma, which reinforced the theory of “one airway, one disease” (10).
However, there have been a paucity of literature reports on NAR, possibly due to the complex and poorly recognized mechanisms. In a multinational cross-sectional study (ECRHS study) investigating the association between rhinitis and asthma, Leynaert and his associates found that rhinitis (AR and NAR) was a risk factor of asthma development, even adjusted for the factors of serum total IgE levels, parental history of asthma and allergen sensitization (11). But it should be recognized that the association between NAR and asthma has not been clearly delineated. In a large-scale longitudinal study, Shaaban and his colleagues found that NAR was associated with asthma development (12). These studies offered significant clues to investigate the impacts of NAR on the lower airways. Many studies have indicated that patients with NAR present asthma and that the inflammation of the airways may be associated with eosinophils (13). However, little is known about lower airway inflammation and responsiveness in patients with NAR without asthma.
Asthma is a chronic airway disease characterized by mucosal inflammation, airway hyperresponsiveness (AHR) and reversible airflow limitation. The lower airway inflammation, as evidenced by induced sputum eosinophilia (14), airflow limitation, non-specific airway hyperresponsiveness (15) and (or) increased levels of fractional exhaled nitric oxide (FeNO) (16), has been shown in patients with AR alone and those with asthma.
Despite the epidemiologic association between NAR and asthma, the relationship between NAR and lower airway inflammation remains elusive in non-asthmatic subjects. It is important to determine whether NAR patients without demonstrated asthma have a lower airways inflammation and the type of inflammation.
In order to study airways inflammation in NAR non-asthmatic subjects, we conducted a double-center study by recruiting non-smokers with NAR (without asthma), non-smokers with AR (without asthma), and healthy subjects. We compared the differences in spirometry, AHR and inflammation.
Methods
Subjects
Between June 2008 and December 2012, we recruited subjects from the department of Otolaryngology, Head and Neck Surgery, Nanjing Jinling Hospital (n=360) and First Affiliated Hospital of Guangzhou Medical University (n=279) consecutively. All subjects were never-smokers. Based on ARIA 2008 criteria (1) and a study by Shaaban et al. (12), patients who had typical nasal symptoms (rhinorrhoea, sneezing, nasal blockage and/or itching, nasal mucosal swelling) were allocated to rhinitis group. Those with positive skin prick test (SPT) findings to the panel of allergens (see below) were defined as AR (n=377), those with negative SPT were defined as NAR (n=262). Nasal polyps and chronic rhinosinusitis were excluded following nasal endoscopy and (or) nasal CT in all patients.
Healthy subjects, free from nasal symptoms, were recruited from the Health Check-up Center. These subjects had no abnormality of physical examinations, blood routine test, chest roentgenography, spirometry and allergen SPT.
For all subjects, the exclusion criteria were: (I) a history of asthma (diagnosed by respiratory physicians), wheezing, chronic cough or other chronic respiratory diseases; (II) acute upper respiratory tract infections within 8 weeks; (III) a history of nasal or facial trauma; (IV) significant nasal septum deviation under endoscopic examination; (V) pregnancy or lactation.
The severity of rhinitis was assessed according to the ARIA 2008 guidelines, moderate-severe rhinitis was diagnosed as having one or more of the following items: abnormal sleep; impairment of daily activities, sport or leisure; problems caused at work or school; troublesome symptoms) (1). Rhinitis that did not fall into the “moderate-severe” category was rated as “mild”.
The protocol was approved by Ethics Committees of Nanjing Jinling Hospital and First Affiliated Hospital of Guangzhou Medical University. All subjects gave written informed consent.
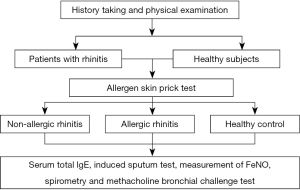
Study flowchart (see Figure 1).
Allergen SPT
Allergen SPT was conducted using standardized kits (AlutardTM, ALK Co. Ltd., Copenhagen, Denmark) containing 13 common allergens. These were Dermatophagoides pteronyssinus, Dermatophagoides farinae, Bloma tropicalis, cat, dog, tree, tree pollen group 1 (plane tree, white poplar, willow and elm), grass pollen 2 (orchard grass, butter Timothy grass and Lolium pasture grass), Blatella germanica, American cockroach, Artemisia argyi, Ambrosia artemisiifolia, mixed mould group 1 (Altanaria tenuis, Chactomium globosum, Cladosporium cladosporoides and Fusarium monilifome) and mixed mould group 2 (Penicillium glaucum, Penicillium expansom and Penicillium notatum). Histamine and natural saline served as the positive and negative control, respectively. All measurements followed the international standardized procedures, with IgE sensitization defined as any wheel size being 3mm greater than the negative control (17).
Total serum IgE assessment
Following phlebotomy, blood samples were collected and sent to the central laboratory for measurement of IgE levels using the Phadiatop UniCAP 100 fluorescent assay instrument (Thermo Scientific Inc., USA). The level of IgE being greater than 150 IU/L was deemed as abnormal (18).
Fractional exhaled nitric oxide (FeNO) assessment
Measurement of FeNO was performed using portable NIOX Mino instruments (Aerocrine Co. Ltd., Sweden), based on international guidelines (19). Nitrogen-rich foods, cola, smoking and exercises were, if any, withheld for at least 2 hours. Subjects in a seated position were instructed to empty their lungs before deep inhalation of the gases free of nitric oxide to total lung capacity through a mouthpiece. This was followed by exhalation at constant airflow (50 mL/s) for 10 s, which entailed an automated program that calculated and displayed reading of FeNO, in parts per billion (ppb). The normative range of FeNO was between 5 and 25 ppb (20), therefore readings greater than 25 ppb were deemed abnormal.
Spirometry
Spirometry was conducted using MasterscreenTM spirometers (Jaeger Co. Ltd., Hochberg, Germany), based on international standardizations (21). Briefly, a minimal of 3 and a maximal of 8 spirometric maneuvers were performed, with differences in FVC of 5% or 200 mL or less between the best two maneuvers. We analyzed the FVC, FEV1, FEV1/FVC, maximal mid-expiratory flow (MMEF), mid-expiratory flow when 50% of FVC has been expired (MEF50%) and mid-expiratory flow when 75% of FVC has been expired (MEF25%). All parameters were recorded as absolute values and percentage per predicted. Normative values were derived from the equations recommended by Zheng et al. (22).
Methacholine bronchial challenge test
Methacholine bronchial challenge test was, based on Yan’s protocol (23), performed with doubling doses of methacholine (Sigma Aldrich Co. Ltd., Ann Arbor, USA) diluents (0.078, 0.156, 0.312, 0.625, 1.25 mg) using the type TAR-1 hand-squeeze nebulizers (Viasys Co. Ltd., Guangzhou, China) (24). Spirometry was measured within 1 min of nebulization, which entailed recording of the FEV1 fall. Bronchial challenge was ceased in case of FEV1 fall being 20% or greater, or the maximal cumulative dose of methacholine (2.50 mg) had been administered. AHR was defined as 20% or greater fall in FEV1 (21).
Induced sputum cytology
Sputum induction was performed based on Chinese Guidelines for the Diagnosis and Management of Cough (25). Before sputum induction, subjects were instructed to fully empty their mouth to remove any cellular debris. Nebulization was conducted using 3%, 4% or 5% saline via ultrasonic nebulizers, as appropriate, with the duration of 5 min for individual concentrations. Following expectoration into clear sterile plastic pot, sputum plugs were weighed and treated with four aliquots of dithiothreitol to completely dissolve mucus. The mixture was subsequently vortexed, shaked and centrifuged to remove supernatants. Cell pallets were mounted on a glass slide for fixation with polyaldehyde and haematoxylin-eosin staining. Samples with 5% or fewer epithelial cells of total cell count were deemed eligible. This entailed counting of 400 non-squamous cells for cytology assessment. Sputum eosinophilia was defined as the proportion of eosinophils being 2.5% or greater (25).
Blinding of assessments
All measurements were performed by designated research technicians who were blinded to study allocation.
Statistical analyses
Statistical analyses were conducted using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Serum total IgE, induced sputum eosinophil count and FeNO were expressed as median (interquartile) because of non-normal distribution, whereas the remaining parameters were presented as mean ± standard deviation. Categorical data were presented as number (percentage) and compared using Fisher’s exact probability chi-square test. Numerical data were compared using independent t-tests or Mann-Whitney tests for two-group comparisons, and one-way analysis of variance or Kruskal-Wallis tests, as appropriate. Spearman’s rank correlations were constructed to assess the relationships between variables. For all comparisons, P value of 0.05 or less denoted statistical significance.
Results
Baseline levels
As shown in Table 1, there were no significant differences in the height, sex, weight and BMI among the three groups. The proportion of patients was similar in AR and NAR.
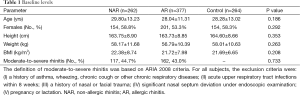
Full table
Spirometry
The FVC and FEV1 predicted did not differ statistically among the three groups (both P>0.05). Compared with healthy subjects, patients with AR were characterized by significantly lower levels of FEV1/FVC, MMEFpred% and MEF25%pred% (all P<0.05), whilst those with NAR presented notably lower levels of FEV1/FVC, MMEFpred%, MEF25%pred% and MEF50%pred% (all P<0.05). However, the differences in spirometric indices between AR and NAR patients were not significant. Group AR had 46 cases of AHR and yielded the highest positivity rate (12.2%) of AHR, followed by group NAR (n=16, 6.1%) and healthy subjects (n=3, 1.1%) (P<0.05) (Table 2).

Full table
Serum total IgE
Serum total IgE was significantly higher in group AR, followed by group NAR, as compared healthy subjects (all P<0.05). However, the serum total IgE levels were numerically but not statistically higher in group NAR than in healthy subjects (Table 3).

Full table
FeNO
Both AR and NAR groups yielded significantly higher FeNO compared with healthy subjects (both P<0.05).
Sputum cytology
Induced sputum eosinophil count was numerically but not statistically higher in group NAR than in healthy subjects. There were no differences for induced sputum neutrophil count among three groups (Table 3).
Total abnormality rate of lower airway indices
In our study, abnormality of lower airways was defined either of the following abnormalities: elevated levels of induced sputum eosinophils, higher FeNO levels, or AHR. It was shown that, compared with healthy subjects, the abnormality rate of lower airways was significantly higher in group AR, followed by group NAR (both P<0.01) (Table 3).
The individual PD20FEV1 values of three groups were not plotted because we calculated these values in subjects with AHR only.
Correlation between airway inflammation and airway hyperresponsiveness (AHR) and spirometry
Patients with rhinitis were divided into AHR group (with AHR) and NAHR group (without AHR) respectively. Table 4 showed that induced sputum Eos and FeNO were both higher in AHR group than in NAHR group with AR (all P<0.05). Median of FeNO was numerically but not statistically higher in AHR group than NAHR group with NAR. Table 5 showed that induced sputum Eos was correlated with MMEF and FeNO in AR (all P<0.05), but the correlation can only be seen between induced sputum Eos and FeNO in NAR (P<0.05).

Full table
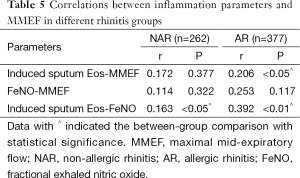
Full table
Discussion
In our study, we found that non-smoked, non-asthmatic patients with NAR yielded markedly heightened lower airway inflammatory responses compared with healthy subjects and that the magnitude of abnormality was lower than patients with AR, suggesting similar changes underlying the airway inflammation between these two clinical phenotypes of rhinitis. Patients with higher FeNO and AHR had poorer pulmonary function. This might offer rationales for early intervention of the upper airway inflammation leading to attenuation or remission of lower airway inflammation.
AR is the upper airway inflammatory disease in which IgE and eosinophils are involved (1). Eosinophils have been recognized as the most crucial inflammatory cells leading to the development of asthma (26). Sputum eosinophilia may be present in patients with AR without asthma and has also been associated with the development of AHR (27). In keeping with literature reports, we found that patients with AR without asthma yielded markedly higher levels of serum IgE, induced sputum eosinophils and FeNO compared with healthy subjects, and that a subgroup of these patients had presented with AHR, further confirming the “one airway, one disease” hypothesis (10).
It should be recognized that, from an epidemiological point of view, the AR and NAR are both strong risk factors of asthma (28-30) irrespective of the atopic status (12). Our study has offered valuable clinical data in support of these epidemiologic findings. Apart from the eosinophilic inflammation observed in NAR with eosinophilic syndrome (NARES), the neurogenic inflammation, including trigeminal nerve reflex, autonomous neurologic disorders (31,32) and abnormal neurologic responses (33), could have played considerable roles in the development of NAR. The only disparity between AR and NAR, when analyzed in terms of their definitions, would have been the presence of atopy. However, similar clinical manifestations underlying AR and NAR have also been reported (17,32). Taken together, a certain degree of similarity between NAR and AR could be confirmed. However, the associations of NAR and lower airway inflammation as well as AHR remain largely unknown.
Small airway dysfunction has been the hallmark of early-stage lung function decline (34,35) and is associated with AHR, reduction in FEV1 and multiple allergen sensitization (36). In this study, we have demonstrated that small airway indices, despite being within the normal range, were significantly lower in patients with NAR than in healthy subjects. Meanwhile, the differences in small airway indices between patients with NAR and those with AR were not statistically significant. These findings collectively suggested a similar magnitude of small airway dysfunction underlying both disorders. As a hallmark of asthma, AHR has been a prerequisite for the diagnosis. It has been reported that 14% to 58% of asymptomatic patients with AHR eventually developed into symptomatic asthma (37,38), suggesting that asymptomatic AHR could be de facto the “subclinical asthma” (39). In this study, the prevalence of AHR was markedly higher in groups NAR and AR compared with healthy subjects (6.1% and 12.2% vs. 1.1%, P<0.05). Our findings pointed to existence of “subclinical asthma” and (or) small airway dysfunction in patients with AR or NAR.
FeNO has been a non-invasive tool for monitoring airway inflammation (40) and is recommended for measurement of lower airway inflammation, asthma diagnosis and efficacy evaluation (19). We noted that groups NAR and AR showed higher levels of FeNO and positivity, despite that the positivity was lower in patients with NAR (48.5%) than in those with AR (59.9%), indicating the similarity between AR and NAR. Furthermore, FeNO has been shown to predict the incidence of AHR in patients with AR (41), and the elevated FeNO has been associated with higher serum IgE levels (42). Our data showed that FeNO value was associated with AHR, MMEF and induced sputum eosinophils in AR patients. Although NAR patients with AHR had high levels of FeNO, it did not show statistic significance. These may contribute to the complicated mechanism of NAR or multiple factors affecting FeNO.
As a specific marker of airway allergic inflammation, eosinophils have been associated with lower AHR and allergic responses. Here, we have demonstrated that markedly more patients with NAR presented with induced sputum eosinophilia (16.4%) than healthy subjects (2.7%), despite that 36.9% of patients with AR indeed showed sputum eosinophilia. It has been reported that NARES accounted for 33% of the NAR (43) and that the NARES with AHR but without lower airway symptoms has been fairly common (46%) (13). Intriguingly, the proportion of patients with sputum eosinophilia was lower in NAR than in AR. A possible interpretation could be that one third of NAR patients harbor nasal and lower airway eosinophilic inflammation and that two thirds of NAR patients might be predominated by the neurogenic inflammation or miscellaneous mechanisms (31,33). Although some part of asthma patients yield airway neutrophilic inflammation, in our data rhinitis patients (whatever NAR or AR) did not showed airway neutrophilic inflammation compared with healthy control. Neutrophilic inflammation may not dominate the main factors of upper and lower airway link.
Our study has some clinical significance. NAR is a disorder partly mimicking AR that has been associated with a similar magnitude of abnormality in terms of lower airway inflammation, AHR and small airway dysfunction, suggesting that physicians should pay more attention to the future risks of developing into asthma, and that early intervention with medications might be associated with a reduced incidence of asthma.
Our major strength was the cross-sectional study conducted in two major specialist centers with large sample size, which was deemed sufficient to power statistical analyses. However, some potential limitations should be underlined. First, the lack of long-term follow-up visits did not allow us to confirm that NAR patients with lower airway inflammation or AHR are more likely to develop asthma than those without. Second, since allergen nasal challenge tests were not performed, the presence and proportion of patients with local allergic rhinitis (LAR) (32) could not be determined. The existence of lower airway inflammation or AHR in patients with predefined LAR warrants further investigation.
In summary, NAR harbors lower airway inflammation characterized by small airway dysfunction, AHR, sputum eosinophilia and increased FeNO which largely mimics that of AR in patients without asthma, despite a lower magnitude of disorders. These findings will offer crucial rationales for early intervention of upper airway inflammation in patients with NAR leading to attenuated lower airway inflammation and future risks of asthma. Further studies confirming these postulations are of merit.
Acknowledgements
Funding: This study was supported by Open Research Foundation of State Key Laboratory of Respiratory Diseases (2007DA780154F0907).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008;63 Suppl 86:8-160. [PubMed]
- Montnémery P, Svensson C, Adelroth E, et al. Prevalence of nasal symptoms and their relation to self-reported asthma andchronic bronchitis/emphysema. Eur Respir J 2001;17:596-603. [PubMed]
- Olsson P, Berglind N, Bellander T, et al. Prevalence of self-reported allergic and non-allergic rhinitis symptoms in Stockholm: relation to age, gender, olfactory sense and smoking. Acta Otolaryngol 2003;123:75-80. [PubMed]
- Bachert C, van Cauwenberge P, Olbrecht J, et al. Prevalence, classification and perception of allergic and nonallergic rhinitis in Belgium. Allergy 2006;61:693-8. [PubMed]
- O’Byrne P, Bateman ED, Bousquet JD, et al. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Updated 2009, Available online: . Accessed August 19th, 2014.http://www.ginasthma.org/Guidelines/guidelines-resources.html
- Settipane RJ, Hagy GW, Settipane GA. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Proc 1994;15:21-5. [PubMed]
- Compalati E, Ridolo E, Passalacqua G, et al. The link between allergic rhinitis and asthma: the united airways disease. Expert Rev Clin Immunol 2010;6:413-23. [PubMed]
- Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 2001;108:S147-334. [PubMed]
- Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol 2010;126:466-76. [PubMed]
- Grossman J. One airway, one disease. Chest 1997;111:11S-16S. [PubMed]
- Leynaert B, Neukirch C, Kony S, et al. Association between asthma and rhinitis according to atopic sensitization in apopulation-based study. J Allergy Clin Immunol 2004;113:86-93. [PubMed]
- Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet 2008;372:1049-57. [PubMed]
- Leone C, Teodoro C, Pelucchi A, et al. Bronchial responsiveness and airway inflammation in patients with nonallergic rhinitis with eosinophilia syndrome. J Allergy Clin Immunol 1997;100:775-80. [PubMed]
- Tatar M, Petriskova J, Zucha J, et al. Induced sputum eosinophils, bronchial reactivity, and cough sensitivity in subjects with allergic rhinitis. J Physiol Pharmacol 2005;56 Suppl 4:227-36. [PubMed]
- Cirillo I, Pistorio A, Tosca M, et al. Impact of allergic rhinitis on asthma: effects on bronchial hyperreactivity. Allergy 2009;64:439-44. [PubMed]
- Lee KJ, Cho SH, Lee SH, et al. Nasal and exhaled nitric oxide in allergic rhinitis. Clin Exp Otorhinolaryngol 2012;5:228-33. [PubMed]
- Rondón C, Romero JJ, López S, et al. Local IgE production and positive nasal provocation test in patients with persistent nonallergic rhinitis. J Allergy Clin Immunol 2007;119:899-905. [PubMed]
- Khan S, Doré PC, Sewell WA. The value of total IgE levels in the context of specific allergy. Pediatr Allergy Immunol 2008;19:777-8. [PubMed]
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602-15. [PubMed]
- Popa V. ATS guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med 2001;163:292-3. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [PubMed]
- Zheng J, Zhong N. Normative values of pulmonary function testing in Chinese adults. Chin Med J (Engl) 2002;115:50-4. [PubMed]
- Yan K, Salome C, Woolcock AJ. Rapid method for measurement of bronchial responsiveness. Thorax 1983;38:760-5. [PubMed]
- Zhong NS, Chen RC. Bronchial hyperresponsiveness in young students of southern China: relation to respiratory symptoms, diagnosed asthma, and risk factors. Thorax 1990;45:860-5. [PubMed]
- Asthma Workgroup of Chinese Society of Respiratory Diseases (CSRD), Chinese Medical, Association The Chinese national guidelines on diagnosis and management of cough. Chin J Tuberc Respir Dis 2009;32:407-13.
- Wardlaw AJ, Brightling C, Green R, et al. Eosinophils in asthma and other allergic diseases. Br Med Bull 2000;56:985-1003. [PubMed]
- Foresi A, Leone C, Pelucchi A, et al. Eosinophils, mast cells, and basophils in induced sputum from patients with seasonal allergic rhinitis and perennial asthma: relationship to methacholine responsiveness. J Allergy Clin Immunol 1997;100:58-64. [PubMed]
- Guerra S, Sherrill DL, Martinez FD, et al. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol 2002;109:419-25. [PubMed]
- Lourenço O, Fonseca AM, Taborda-Barata L. Asthma is more frequently associated with non-allergic than allergic rhinitis in Portuguese patients. Rhinology 2009;47:207-13. [PubMed]
- Leynaert B, Bousquet J, Neukirch C, et al. Perennial rhinitis: An independent risk factor for asthma in nonatopic subjects: results from the European Community Respiratory Health Survey. J Allergy Clin Immunol 1999;104:301-4. [PubMed]
- Scichilone N, Battaglia S, Taormina S, et al. Alveolar nitric oxide and asthma control in mild untreated asthma. J Allergy Clin Immunol 2013;131:1513-7. [PubMed]
- Rondón C, Campo P, Togias A, et al. Local allergic rhinitis: concept, pathophysiology, and management. J Allergy Clin Immunol 2012;129:1460-7. [PubMed]
- Silvers WS. The skier’s nose: a model of cold-induced rhinorrhea. Ann Allergy 1991;67:32-6. [PubMed]
- Cirillo I, Klersy C, Marseglia GL, et al. Role of FEF25%-75% as a predictor of bronchial hyperreactivity in allergic patients. Ann Allergy Asthma Immunol 2006;96:692-700. [PubMed]
- Ciprandi G, Cirillo I, Klersy C, et al. Role of FEF25-75 as an early marker of bronchial impairment in patients with seasonal allergic rhinitis. Am J Rhinol 2006;20:641-7. [PubMed]
- Ciprandi G, Cirillo I, Klersy C. Lower airways may also be affected in asymptomatic patients with recent onset of allergic rhinitis. Laryngoscope 2010;120:1288-91. [PubMed]
- Hopp RJ, Townley RG, Biven RE, et al. The presence of airway reactivity before the development of asthma. Am Rev Respir Dis 1990;141:2-8. [PubMed]
- Laprise C, Laviolette M, Boutet M, et al. Asymptomatic airway hyperresponsiveness: relationships with airway inflammation and remodelling. Eur Respir J 1999;14:63-73. [PubMed]
- Zhong NS, Chen RC, Yang MO, et al. Is asymptomatic bronchial hyperresponsiveness an indication of potential asthma? A two-year follow-up of young students with bronchial hyperresponsiveness. Chest 1992;102:1104-9. [PubMed]
- Barnes PJ, Dweik RA, Gelb AF, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest 2010;138:682-92. [PubMed]
- Cirillo I, Ricciardolo FL, Medusei G, et al. Exhaled nitric oxide may predict bronchial hyperreactivity in patients with allergic rhinitis. Int Arch Allergy Immunol 2013;160:322-8. [PubMed]
- Cardinale F, de Benedictis FM, Muggeo V, et al. Exhaled nitric oxide, total serum IgE and allergic sensitization in childhood asthma and allergic rhinitis. Pediatr Allergy Immunol 2005;16:236-42. [PubMed]
- Wang ZY. Comparison on the clinical characteristics of allergic rhinitis and non-allergic rhinitis. Chin J Otorhinol Skull base Surg 2010;17:340-3.