Anti-angiogenic agents in Non-Small-Cell Lung Cancer (NSCLC): a perspective on the MONET1 (Motesanib NSCLC Efficacy and Tolerability) study
Since the discovery of the critical role played by angiogenesis in tumor growth and development (1), there has been increased interest in evaluating anti-angiogenic agents in the treatment of various malignancies, including non-small-cell lung cancer (NSCLC). Bevacizumab is a humanized monoclonal antibody to vascular endothelial growth factor (VEGF) and is approved for use in combination with chemotherapy in multiple countries for the treatment of patients with metastatic NSCLC. Approvals were based upon an improvement in response rate (RR) and progression-free survival (PFS) seen with the addition of bevacizumab to chemotherapy in two large phase III studies, the North American Eastern Cooperative Oncology Group (ECOG) 4599 (2) and the European AVAiL (3). Additionally, an overall survival (OS) benefit was seen in ECOG 4599 among those patients receiving bevacizumab. The exciting results seen with bevacizumab have led to the development and exploration of other anti-angiogenic agents, including small molecule tyrosine kinase inhibitors (TKIs) targeting the VEGF receptor (VEGFR).
Advantages of anti-angiogenic TKIs include the ability to target multiple receptor pathways and oral availability. After the success of E4599 and AVAiL utilizing bevacizumab, several phase III randomized trials of VEGFR TKIs administered either alone or in combination with chemotherapy have been conducted in NSCLC (Table 1). Despite improvements in RR or PFS noted in a number of these trials, none of the trials conducted so far have demonstrated an OS benefit. The lack of predictive biomarkers continues to be a major hurdle that must be overcome in order to make real steps forward in our use of anti-angiogenic therapies for NSCLC and other malignancies.
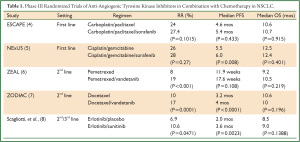
Full table
Motesanib is a selective oral inhibitor of VEGF receptors-1, 2, and 3, platelet-derived growth factor receptor (PDGFR), and c-Kit that initially demonstrated promising antitumor activity in trials of thyroid cancer (9,10). A phase IB study of motesanib in advanced refractory tumors established a maximum tolerated dose (MTD) of 125 mg once daily, with five patients (7%) achieving a partial response with the drug as a single agent (11). A randomized phase II trial of motesanib or bevacizumab in combination with carboplatin/paclitaxel as frontline therapy for patients with advanced NSCLC found that RR, PFS, and OS were comparable in those patients receiving either motesanib 125 mg daily (n=61) or bevacizumab 15 mg/kg intravenously every 3 weeks (n=63) (12). A subsequent biomarker analysis discovered a strong association between an increase in placental growth factor (PlGF) during the first three weeks of treatment and therapeutic response to treatment with motesanib (13). PlGF acts as a VEGF homolog by promoting angiogenesis through VEGFR-1 activation (14).
These encouraging results, including a potential biomarker to predict efficacy, led to the development of the Motesanib NSCLC Efficacy and Tolerability (MONET1) trial, an international, phase III randomized, placebo-controlled study of patients with advanced non-squamous NSCLC who received frontline carboplatin/paclitaxel either alone or in combination with motesanib at 125 mg daily. The results of this study were published in the August 10, 2012 issue of the Journal of Clinical Oncology by Dr. Giorgio Scagliotti and colleagues (15). The primary endpoint of this trial was OS, with secondary endpoints of PFS, overall response rate (ORR), adverse events, (AEs), and association between PlGF change and OS. Initially, the study allowed all histologic subtypes, but the protocol was later amended (after enrollment of 360 patients with squamous histology) to include only those patients with non-squamous histology. This restriction was due to an increased rate of hemoptysis and mortality seen in patients with squamous histology, similar to toxicities seen in the patient population with other VEGF targeted agents such as bevacizumab. A total of 1,090 patients with non-squamous NSCLC were randomized to receive motesanib (n=541; arm A) or placebo (n=549, arm B) in combination with carboplatin and paclitaxel as first-line NSCLC therapy. Although patients receiving motesanib had an improvement in median PFS (5.6 versus 5.2 months, P<0.001) and ORR (40% versus 26%, P<0.001), there was not a statistically significant difference in median OS between the two arms (13.0 versus 10.0 months, HR: 0.90, 95% CI 0.78-1.04, P=0.14). Moreover, there was no association between PlGF change and OS in arm A, despite the association seen in the phase II study. The incidence of AEs was also greater in those patients receiving motesanib, compared to chemotherapy alone, including grade >/= 3 AEs (73% and 59%, arms A and B, respectively) and grade 5 AEs (14% and 9%, respectively). Interestingly, prespecified subgroup analyses indicated that patients with adenocarcinoma and of Asian descent may preferentially benefit from motesanib. Overall, however, these results add to the expanding list of negative studies involving VEGFR TKIs added to chemotherapy in unselected patients with NSCLC.
What are some of the reasons that trials of VEGFR TKIs are failing to show significant benefit in NSCLC? First, adequate patient selection remains a critical issue and can only be achieved through the identification of reliable predictive biomarkers, which has remained an elusive goal. Plasma biomarkers of angiogenesis have been studied extensively in prior trials, including VEGF levels. In ECOG 4599, high plasma VEGF levels correlated with higher response to bevacizumab, but did not predict for a survival benefit (16). In a NSCLC study of vandetanib, another VEGFR TKI, low levels of VEGF were found to be associated with an improvement in PFS (17). Marker signatures that allow profiling of multiple plasma markers have also been studied and correlated with response to VEGFR TKIs. An analysis of 33 patients treated with pazopanib found that cytokine and angiogenic factor (CAF) profiles were associated with tumor shrinkage, primarily seen with changes in soluble VEGFR-2 and IL-4, and with baseline levels of IL-12 and hepatocyte growth factor (HGF) (18). Despite these associations, the cytokines of interest have been variable with other VEGFR-TKIs in studies by the same group (19) and others (20).
Genetic variants in the VEGF pathway, including small nucleotide polymorphisms (SNPs), as well as numerous other angiogenic markers, such as intracellular adhesion molecule (ICAM), basic fibroblast growth factor (bFGF), and PlGF, have also been evaluated previously, though the results obtained to date have not been reliable or validated and are thus not ready for clinical use (21,22). While a significant association was seen between PlGF levels and response to motesanib in the preceding phase II study, disappointingly, this finding was not confirmed in the current phase III study. Asian patients did appear to preferentially benefit from motesanib in this trial, but a genetic marker to explain this result has yet to be elucidated. Although it has been a frustrating effort to date, there remains hope for the discovery of angiogenic biomarkers that will aid in patient selection for future trials of VEGFR TKIs.
Other reasons for the lack of survival benefit seen with the addition of multi-targeted TKIs to chemotherapy include possible antagonism between VEGFR TKIs and chemotherapy, as well as the development of AEs that may limit the therapeutic effect of these drugs. A previous study demonstrated that cell cycle arrest in the G1 phase occurs with sunitinib, an inhibitor of VEGFR-1, -2, -3, PDGFR alpha/beta, c-kit, Flt-3, and RET, which impairs the efficacy of concurrent or sequential treatment with docetaxel (23). Thus, it remains unknown whether the VEGFR TKIs in development should be evaluated as single agents or in combination with chemotherapy given this potential antagonism. Also, as they are often multi-targeted, there is concern for the development of additive AEs that could hinder the achievement of a therapeutic dose of the VEGFR-TKIs, especially in combination with chemotherapy. Though a high rate of AEs was seen in MONET1, it seems unlikely that a failure to reach therapeutic drug levels would explain the lack of a survival benefit as only 150 patients (28.2%) discontinued motesanib due to an adverse event, with the majority of patients receiving the full 125 mg dose known to be effective as a single agent. It is also possible that the tumors are able to develop bypass pathways to circumvent the VEGF inhibition and that we have yet to fully understand the alternative pathways related to angiogenesis.
In summary, the MONET1 study is another phase III trial that failed to achieve its primary endpoint of OS when adding a VEGFR TKI to first-line chemotherapy. Though significant improvements in response and PFS were reported, those came at a cost of increased toxicity, and without an OS benefit. This study then joins an expanding list of negative trials evaluating the addition of a VEGFR TKI to chemotherapy in patients with NSCLC. Further exploration of VEGFR TKIs in an unselected patient population is unlikely to provide positive efficacy data, especially when given in combination with first-line platinum doublet chemotherapy. The identification of predictive angiogenic biomarkers and a better understanding of the complexities related to multiple pathway inhibition and interactions with chemotherapy remain important goals for investigators as these agents continue to be studied in NSCLC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6.
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50.
- Reck M, von Pawel J, Zatlouka P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227-34.
- Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 2010;28:1835-42.
- Paz-Ares LG, Biesma B, Heigener D, et al. Phase III, Randomized, Double-Blind, Placebo-Controlled Trial of Gemcitabine/Cisplatin Alone or With Sorafenib for the First-Line Treatment of Advanced, Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2012;30:3084-92.
- de Boer RH, Arrieta Ó, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol 2011;29:1067-74.
- Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 2010;11:619-26.
- Scagliotti GV, Krzakowski M, Szczesna A, et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J Clin Oncol 2012;30:2070-8.
- Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 2008;359:31-42.
- Schlumberger MJ, Elisei R, Bastholt L, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol 2009;27:3794-801.
- Rosen LS, Kurzrock R, Mulay M, et al. Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol 2007;25:2369-76.
- Blumenschein GR Jr, Kabbinavar F, Menon H, et al. A phase II, multicenter, open-label randomized study of motesanib or bevacizumab in combination with paclitaxel and carboplatin for advanced nonsquamous non-small-cell lung cancer. Ann Oncol 2011;22:2057-67.
- Bass MB, Sherman SI, Schlumberger MJ, et al. Biomarkers as predictors of response to treatment with motesanib in patients with progressive advanced thyroid cancer. J Clin Endocrinol Metab 2010;95:5018-27.
- Autiero M, Luttun A, Tjwa M, et al. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost 2003;1:1356-70.
- Scagliotti GV, Vynnychenko I, Park K, et al. International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin Oncol 2012;30:2829-36.
- Dowlati A, Gray R, Sandler AB, et al. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab--an Eastern Cooperative Oncology Group Study. Clin Cancer Res 2008;14:1407-12.
- Hanrahan EO, Ryan AJ, Mann H, et al. Baseline vascular endothelial growth factor concentration as a potential predictive marker of benefit from vandetanib in non-small cell lung cancer. Clin Cancer Res 2009;15:3600-9.
- Nikolinakos PG, Altorki N, Yankelevitz D, et al. Plasma cytokine and angiogenic factor profiling identifies markers associated with tumor shrinkage in early-stage non-small cell lung cancer patients treated with pazopanib. Cancer Res 2010;70:2171-9.
- Hanrahan EO, Lin HY, Kim ES, et al. Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. J Clin Oncol 2010;28:193-201.
- Zurita AJ, Jonasch E, Wang X, et al. A cytokine and angiogenic factor (CAF) analysis in plasma for selection of sorafenib therapy in patients with metastatic renal cell carcinoma. Ann Oncol 2012;23:46-52.
- Lambrechts D, Claes B, Delmar P, et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: an analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol 2012;13:724-33.
- Zhang W, Dahlberg SE, Yang D, et al. Genetic variants in angiogenesis pathway associated with clinical outcome in NSCLC patients (pts) treated with bevacizumab in combination with carboplatin and paclitaxel: Subset pharmacogenetic analysis of ECOG 4599. J Clin Oncol 2009;27:15s (abstr 8032).
- Pan F, Tian J, Zhang X, et al. Synergistic interaction between sunitinib and docetaxel is sequence dependent in human non-small lung cancer with EGFR TKIs-resistant mutation. J Cancer Res Clin Oncol 2011;137:1397-408.


