Cardiopulmonary resuscitation using electrically driven devices: a review
In Europe 40% of all deaths in patients 75 years or younger are related to cardiovascular disease. Among these, sudden cardiac arrest (SCA) is a medical emergency, most often caused by coronary artery disease. The incidence of an out-of-hospital cardiac arrest (OHCA) in Europe is 38 per 100,000 inhabitants per year (1). The incidence of in hospital cardiac arrest (IHCA) varies between 1-5 cases per 1,000 admissions.
In most cases of SCA, pulseless ventricular tachycardia (VT) or ventricular fibrillation (VF) is the initial rhythm. Starting cardiopulmonary resuscitation (CPR) including cardiac defibrillation may result in return of spontaneous circulation (ROSC) (2). If not treated immediately, a depletion of adenosine triphosphate (ATP) will occur with consecutive conversion into asystole or pulseless electric activity (PEA) (3).
Depending on the initial heart rhythm, survival rates to hospital discharge may vary widely. About 21.2% of people suffering SCA may survive if the initial rhythm is VF or VT (4). In contrast, if the initial rhythm is asystole or PEA, only 11.5% of in hospital SCA will survive to hospital discharge.
The first goal of resuscitation is to reestablish sufficient circulation to supply brain and vital organs with oxygen. Chest compressions and external defibrillation are the first line of external circulatory support. Kouwenhoven provided the first clinical evidence of efficacy of external manual chest compressions in 1960 (5). Over the course of time, guidelines were developed and modified regarding frequency, compression depth and ratio of ventilation to chest compression. The European Resuscitation Council (ERC) and the American Heart Association (AHA) continuously edit these guidelines (6,7). Although early defibrillation is the most important factor influencing survival, nearly every resuscitation requires external chest compressions as well. The basic principle of external chest compression is rhythmical administration of a force, either punctual to the sternum or circumferential around the chest, to generate blood flow by increasing intrathoracic pressure. Two theories are based on this principle: the heart pump theory (8) and the thoracic pump theory (9). The heart pump theory postulates that blood flow during resuscitation is achieved by direct compression of the heart against the spine. The thoracic pump theory assumes that blood flow is induced by indirect compression and decompression of the heart through changes of intrathoracic pressure. During decompression, the chest should be allowed to recoil completely to provide adequate filling of the heart (6,7).
A key determinant in CPR to reestablish organ perfusion is the efficacy of external chest compressions. Sufficient perfusion of the coronary arteries is needed to restore ATP levels in the myocardium and thereby increase chances for successful defibrillation. Coronary perfusion pressures (CPP) above 15 mmHg are associated with a higher incidence of ROSC (10-12).
The gold standard in external chest compressions during resuscitation is manual compressions with a frequency between 100 and 120 compressions/min and a compression depth of 5 cm. However, several studies showed that even professional rescuers are not able to perform high quality chest compressions over a longer period of time without tiring. Abella and colleagues showed that chest compressions performed during in-hospital resuscitation did not meet the recommended frequency in 30% of the cases and not the required depth in 40% (13). Weariness starts approximately after 1 min of CPR with a continuous drop in quality afterwards. During the first 3 min, adequate compressions with regard to depth and frequency decreased significantly from 92% to 67% and 39%, respectively, and even to 18% after 5 min (14). This underlines that effectiveness of manual chest compressions is to a great extend influenced by the rescuer's endurance. As a consequence, recommendations are to alternate between rescuers every 2 min during chest compressions, if possible (6,7). In addition, manual resuscitation approximately provides only a third of the regular blood flow to the brain and 20% of the regular blood flow to the heart (15).
Another major drawback of manual CPR is the need to interrupt chest compressions, i.e., for defibrillation or during transport. This leads to an accumulation of no-flow time. A direct inverse relationship exists between duration of chest compression interruptions and short-term survival (16,17), caused by inadequate cerebral and coronary perfusion (18).
The use of mechanical resuscitation devices offers a potential solution to minimize these periods of no flow and to provide improved systemic and coronary perfusion in comparison to manual chest compressions. In 1908, Pike provided the first description of a machine performing rhythmical chest compressions in animals (19). The first mechanically driven chest “presses” were introduced in the 1960’s (20-22). Since then, a steady development has taken place leading to more lightweight and user friendly devices with the idea to provide constant high-quality chest compressions while minimizing periods of no flow. In an experimental setting, Rubertsson and colleagues provided evidence that mechanical resuscitation with a Lund University Cardiac Assist System (LUCAS) 1 device resulted in significantly improved cortical cerebral blood flow and end- tidal CO2 concentration compared to manual chest compressions (23). Liao and coworkers were able to demonstrate significantly higher CPP in the mechanical resuscitation group with CPP of 20 mmHg compared to manual chest compressions with CPP of 5 mmHg (24). Wagner and colleagues verified a good correlation between CPP and average peak coronary flow velocity as time-averaged value of the instantaneous peak velocity samples over the last 2 cardiac cycles in centimeters per second. In their experimental setting they used a porcine model for CPR with a LUCAS device and achieved even higher coronary blood flow velocities with mechanical chest compressions compared to baseline, defined as measurement after induction of anesthesia and completion of instrumentation (25).
In this review we will focus on two modern tools already in clinical use: LUCAS (Physio Control, Redmond, WA, USA) and the AutoPulse Device (ZollMedical, Chelmsford, USA).
The Lund University Cardiac Assist System (LUCAS)
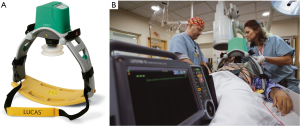
The LUCAS I device consists of a pneumatic cylinder mounted on two legs, connected to a stiff back plate. A silicone rubber suction cup attached to a pneumatic cylinder transfers the force to the chest, thereby providing active compression and decompression (26). A fully pneumatic driven device was first launched in Europe in 2003 and later, after some modifications, also in the US, Japan and other countries. Due to laborious handling with the gas cylinder and a high consumption of compressed air, a second generation device of the LUCAS was introduced and launched in 2009 as a fully electrical operated device (Figure 1). The compression frequency and depth are consistent with the guidelines (6,7) and allow complete recoil of the chest after compression.
An overview of relevant literature pertaining to the LUCAS device is given in Table 1. Currently there are only two randomized controlled trials (RCT) investigating the effect of the LUCAS device. The LINC trial (LUCAS in cardiac arrest) was conducted as a multicenter RCT between 2008 and 2013 and provides data of 2,589 patients divided in two subgroups (29). A total of 1,300 patients were included to undergo resuscitation with a LUCAS device, compared to 1,289 patients being resuscitated manually. Inclusion criteria were: adult patients suffering from an unexpected non-traumatic OHCA. Exclusion criteria were cardiac arrest due to trauma, age under 18, known pregnancy and physiologic constitution unsuitable for application of the LUCAS device. Primary objective was the assessment of an advantage in 4-h survival after successful ROSC within the group undergoing mechanical CPR. Secondary endpoints were: ROSC, arrival at emergency department and neurological outcome after discharge from intensive care unit, hospital discharge and 1 and 6 months after the event. Both groups underwent manual chest compressions during the process of randomization. Within the group of patients allocated to mechanical resuscitation, manual CPR was continued until the LUCAS was installed. Within the group of patients receiving only manual CPR, CPR was performed according to the 2005 ERC guidelines. For the primary outcome of 4-h survival after ROSC, no significant difference (P<0.99) was observed between patients in the mechanical CPR group (307/1300; 23.6%) and patients undergoing manual CPR (305/1,289; 23.7%). Additionally, no significant differences for any of the secondary endpoints were observed.
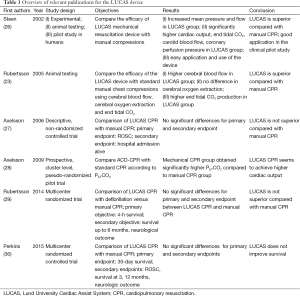
Full table
A second RCT, the Prehospital Randomised Assessment of Mechanical Compression Device in Cardiac Arrest (PaRaMeDIC) study (30), was published recently. This trial was conducted to evaluate the effects of mechanical CPR using the LUCAS-2 on mortality and morbidity in patients suffering from OHCA. A total of 4,471 patients were included in the study: 1,652 assigned to the LUCAS-2 group and 2,819 to manual resuscitation. The primary endpoint was 30-day survival after cardiac arrest. Secondary endpoints were: survived event (ROSC), survival at 3 and 12 months and survival with favorable neurologic outcome at 3 months, defined by Cerebral Performance Category score of 1 or 2. Participating emergency vehicles were randomized in either vehicles carrying a LUCAS-2 device or vehicles performing manual CPR. Inclusion criteria were: adult patients suffering from non- traumatic OHCA. Survival after 30 days was similar between both groups with 104/1,652 patients (6%) in the LUCAS-2 group and 193/2,819 patients. in the control group. No significant differences for secondary endpoints were detected.
As to the potential risk for injuries to the ribs, sternum or internal organs (e.g., liver rupture) for patients undergoing CPR, either manually or with a LUCAS device, recent studies did not show any differences between manual resuscitation and mechanical compression (31-33). Although fatal complications using a LUCAS device have been reported (34,35), the overall incidence compared to manual resuscitation is not increased. Nevertheless it is mandatory to check the correct anatomic positioning of the device during the process of mechanical resuscitation regularly.
AutoPulse
The AutoPulse device consists of a backboard with an attached motor and a load-distributing band (LDB) (Figure 2). Ruled by microprocessors, the device provides chest compressions at a fixed rate of 80 compressions/min and is able to achieve a compression depth of 20-30% of the thoracic circumference. A motor tightens or loosens the LDB while minimizing local stress by distributing the compressive load over the chest.

An overview of relevant literature pertaining to the AutoPulse device is given in Table 2. In animal models, improved coronary and systemic perfusion was achieved by the LDB compared to manual CPR by two mechanisms: first through direct cardiac compressions and, secondly, by increased intrathoracic pressures, generated through airway collapse (41). A direct inverse relationship between the amount of expired air and intrathoracic pressure could be observed (36). A smaller study showed improved survival after LDB-resuscitation in out-of-hospital settings in 162 patients undergoing either mechanical or manual resuscitation (37). In 2006, Ong and colleagues published a phased, non-randomized, observational single center study to compare survival rates among patients with OHCA receiving CPR either manually or with the use of the AutoPulse (39). Between 2001 and 2003, 499 patients suffering from cardiac arrest received manual CPR. From 2003 to 2005, CPR with the AutoPulse was performed in 210 patients. Primary endpoint was ROSC. Secondary endpoints were survival to hospital admission, survival to hospital discharge and neurological and functional status at discharge. Rates of ROSC and survival improved after the introduction of a LDB. With manual CPR, in 20.2% of the patients ROSC could be achieved compared to 34.5% with LDB-CPR. Survival to hospital admission improved (11.1% vs. 20.9%) as well as survival to hospital discharge (2.9% vs. 9.7%). However, no significant difference with regard to neurological and functional status was reported within the group of survivors discharged from hospital.
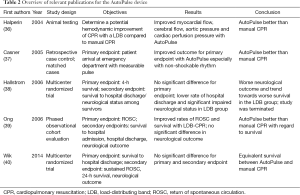
Full table
A multicenter, randomized trial performed by Hallstrom et al. also compared manual CPR to LDB-CPR (38). Among 554 patients assigned to the LDB group and 517 to the manual CPR group, no significant difference in survival at 4 hours was depicted (29.5% vs. 28.5%; P=0.74). The LDB group showed worse outcomes with a lower rate of hospital discharge (5.8% vs. 9.9%, P=0.04) and reduced survival with unaffected neurological status (3.1% vs. 7.5%, P=0.006). Due to the worse outcome of patients in the LDB group, the trial was terminated in 2005.
In 2014, a large multicenter RCT was published, comparing LDB-CPR to manual CPR (40). The Circulation Improving Resuscitation Care (CIRC) trial included a total of 4,231 patients, divided into the LDB-CPR cohort (2,099 patients) and the manual CPR cohort (2,032 patients). Primary endpoint was survival to hospital discharge. Secondary endpoints were defined as sustained ROSC, 24-h survival and modified Rankin Score prior to discharge. Criteria for inclusion were age over 18 and OHCA with presumed cardiac origin. Criteria for exclusion were pregnancy, a “do not resuscitate order”, body size too big for the device, status of being a prisoner, mechanical chest compressions prior to randomization and arrival on scene more than 16 min after the emergency call. In regards to the primary endpoint, 11% of the patients in the manual CPR group survived to hospital discharge compared to 9.4% in the LDB group, the difference not being statistically significant. In addition, no significant difference was observed with regard to neurologic outcome. Hence, the study demonstrated that CPR with AutoPulse is not superior to manual CPR.
Injuries induced by the AutoPulse device were similar to manual CPR (42). However, severe injuries to the liver or other internal organs have been reported in different case reports (43).
Discussion
In the treatment of SCA significant improvements have been achieved recently, but mortality and morbidity rates still remain high (3,44). The recommended treatment is immediate CPR by rhythmical chest compressions and ventilation to achieve reperfusion thereby restoring levels of ATP and supplying oxygen to brain and heart. One major factor which influences the outcome of CPR, such as the time delay between patient collapse and initiation of CPR, cannot be influenced. If this time is prolonged, heart rhythm may convert from VT/VF into asystole/PEA, resulting in poor patient outcome (45). Several studies showed that continuous chest compressions with adequate frequency and compression depth are crucial because circulatory pressure immediately drops if chest compressions are discontinued (13-15,46). Nevertheless, situations will occur in every CPR demanding discontinuation of chest compressions, i.e., rhythm analysis before defibrillation, defibrillation itself, patient transport or other diagnostic or therapeutic interventions. Additionally, it was shown that quality of manual CPR is inconsistent and decreases over time, as rescue personnel gets tired within short periods of time (47). These shortcomings of manual CPR could theoretically be overcome by mechanical resuscitation devices which generate a constant pressure, frequency and compression depth and which may minimizing no flow times. Since both devices, LUCAS and AutoPulse may also be used in catheter labs unnecessary exposure of medical personal to X rays can be avoided.
For both devices, LUCAS and AutoPulse, experimental data are available showing improved coronary and systemic circulation and higher rates of ROSC compared to standard resuscitation (23-25,48). However, large RCT as the LINC- (29) and PARAMEDIC-trial (30) for LUCAS and CIRC trial (39) for AutoPulse were not able to demonstrate a superiority of mechanical resuscitation over manual CPR. However, the quality of clinical trials regarding the efficacy of mechanical resuscitation devices may be limited because of the clinical heterogeneity in respect to the primary endpoints (49). The current results of the CRT regarding the efficacy of the LUCAS device are reflected in the actual guidelines of the AHA with a recommendation level IIb and evidence level (LOE) C (50). Similar to the LUCAS device, there is insufficient evidence for the superiority of LDB devices, reflected by a class IIb recommendation with LOE C in the AHA guidelines (50).
New devices have been developed with modifications in design and setup to improve the quality of CPR and overcome disadvantages associated with other devices. The Corpuls CPR (GS Elektromedizinische Geräte, Kaufering, Germany) will be launched in 2015 (Figure 3). A piston mounted on a Corpuls CPR arm is attached to a resuscitation board to provide chest compression. The arm is adjustable in height, allowing optimal fitting to each individual patient and easy access for therapeutic interventions. Electrically driven, the device can be set to perform between 80 and 120 chest compressions/min, available in three different set-ups (15:2; 30:2; continuous compressions) with an adjustable compression depth between 2 and 6 cm. The Corpuls CPR is available with three different types of resuscitation boards, designed for catheter labs and air and ground ambulances, respectively. Up to now, no literature and only prototypes are available.

Conclusions
In conclusion, current studies failed to demonstrate an improved clinical outcome when using mechanical resuscitation devices compared to manual CPR. Therefore a generalized recommendation for the use of mechanical resuscitation devices cannot be given.
Acknowledgements
The author wants to acknowledge the contribution of Anna Berkefeld in revising the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zheng ZJ, Croft JB, Giles WH, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation 2001;104:2158-63. [PubMed]
- van Alem AP, Vrenken RH, de Vos R, et al. Use of automated external defibrillator by first responders in out of hospital cardiac arrest: prospective controlled trial. BMJ 2003;327:1312. [PubMed]
- Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA 2002;288:3035-8. [PubMed]
- Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008;300:1423-31. [PubMed]
- Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed-chest cardiac massage. JAMA 1960;173:1064-7. [PubMed]
- Nolan JP, Soar J, Zideman DA, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation 2010;81:1219-76. [PubMed]
- Field JM, Hazinski MF, Sayre MR, et al. Part 1: executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122:S640-56. [PubMed]
- Maier GW, Tyson GS Jr, Olsen CO, et al. The physiology of external cardiac massage: high-impulse cardiopulmonary resuscitation. Circulation 1984;70:86-101. [PubMed]
- Rich S, Wix HL, Shapiro EP. Clinical assessment of heart chamber size and valve motion during cardiopulmonary resuscitation by two-dimensional echocardiography. Am Heart J 1981;102:368-73. [PubMed]
- Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 1990;263:1106-13. [PubMed]
- Ralston SH, Voorhees WD, Babbs CF. Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: improved regional blood flow and resuscitation in dogs. Ann Emerg Med 1984;13:79-86. [PubMed]
- Michael JR, Guerci AD, Koehler RC, et al. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation 1984;69:822-35. [PubMed]
- Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA 2005;293:305-10. [PubMed]
- Hightower D, Thomas SH, Stone CK, et al. Decay in quality of closed-chest compressions over time. Ann Emerg Med 1995;26:300-3. [PubMed]
- Kern KB. Coronary perfusion pressure during cardiopulmonary resuscitation. Baillieres Clin Anaesthesiol 2000;14:591-609.
- Kern KB, Hilwig RW, Berg RA, et al. Importance of continuous chest compressions during cardiopulmonary resuscitation: improved outcome during a simulated single lay-rescuer scenario. Circulation 2002;105:645-9. [PubMed]
- Yu T, Weil MH, Tang W, et al. Adverse outcomes of interrupted precordial compression during automated defibrillation. Circulation 2002;106:368-72. [PubMed]
- Ewy GA. Cardiocerebral resuscitation: the new cardiopulmonary resuscitation. Circulation 2005;111:2134-42. [PubMed]
- Pike FH, Guthrie CC, Stewart GN. Studies in resuscitation: i. the general conditions affecting resuscitation, and the resuscitation of the blood and of the heart. J Exp Med 1908;10:371-418. [PubMed]
- Pearson JW, Navarro RN, Redding JS. Evaluation of mechanical devices for closed-chest cardiac massage. Anesth Analg 1966;45:590-8. [PubMed]
- Safar P, Harris LC Jr. The beck-rand external cardiac compression machine. Anesthesiology 1963;24:586-8. [PubMed]
- Nachlas MM, Siedband MP. A simple portable pneumatic pump for external cardiac massage. Am J Cardiol 1962;10:107-9. [PubMed]
- Rubertsson S, Karlsten R. Increased cortical cerebral blood flow with LUCAS; a new device for mechanical chest compressions compared to standard external compressions during experimental cardiopulmonary resuscitation. Resuscitation 2005;65:357-63. [PubMed]
- Liao Q, Sjöberg T, Paskevicius A, et al. Manual versus mechanical cardiopulmonary resuscitation. An experimental study in pigs. BMC Cardiovasc Disord 2010;10:53. [PubMed]
- Wagner H, Madsen Hardig B, Steen S, et al. Evaluation of coronary blood flow velocity during cardiac arrest with circulation maintained through mechanical chest compressions in a porcine model. BMC Cardiovasc Disord 2011;11:73. [PubMed]
- Steen S, Liao Q, Pierre L, et al. Evaluation of LUCAS, a new device for automatic mechanical compression and active decompression resuscitation. Resuscitation 2002;55:285-99. [PubMed]
- Axelsson C, Nestin J, Svensson L, et al. Clinical consequences of the introduction of mechanical chest compression in the EMS system for treatment of out-of-hospital cardiac arrest-a pilot study. Resuscitation 2006;71:47-55. [PubMed]
- Axelsson C, Karlsson T, Axelsson AB, et al. Mechanical active compression-decompression cardiopulmonary resuscitation (ACD-CPR) versus manual CPR according to pressure of end tidal carbon dioxide (P(ET)CO2) during CPR in out-of-hospital cardiac arrest (OHCA). Resuscitation 2009;80:1099-103. [PubMed]
- Rubertsson S, Lindgren E, Smekal D, et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest: the LINC randomized trial. JAMA 2014;311:53-61. [PubMed]
- Perkins GD, Lall R, Quinn T, et al. Mechanical versus manual chest compression for out-of-hospital cardiac arrest (PARAMEDIC): a pragmatic, cluster randomised controlled trial. Lancet 2015;385:947-55. [PubMed]
- Smekal D, Lindgren E, Sandler H, et al. CPR-related injuries after manual or mechanical chest compressions with the LUCAS™ device: a multicentre study of victims after unsuccessful resuscitation. Resuscitation 2014;85:1708-12. [PubMed]
- Smekal D, Johansson J, Huzevka T, et al. No difference in autopsy detected injuries in cardiac arrest patients treated with manual chest compressions compared with mechanical compressions with the LUCAS device--a pilot study. Resuscitation 2009;80:1104-7. [PubMed]
- Oberladstaetter D, Braun P, Freund MC, et al. Autopsy is more sensitive than computed tomography in detection of LUCAS-CPR related non-dislocated chest fractures. Resuscitation 2012;83:e89-90. [PubMed]
- de Rooij PP, Wiendels DR, Snellen JP. Fatal complication secondary to mechanical chest compression device. Resuscitation 2009;80:1214-5. [PubMed]
- Camden JR, Carucci LR. Liver injury diagnosed on computed tomography after use of an automated cardiopulmonary resuscitation device. Emerg Radiol 2011;18:429-31. [PubMed]
- Halperin HR, Paradis N, Ornato JP, et al. Cardiopulmonary resuscitation with a novel chest compression device in a porcine model of cardiac arrest: improved hemodynamics and mechanisms. J Am Coll Cardiol 2004;44:2214-20. [PubMed]
- Casner M, Andersen D, Isaacs SM. The impact of a new CPR assist device on rate of return of spontaneous circulation in out-of-hospital cardiac arrest. Prehosp Emerg Care 2005;9:61-7. [PubMed]
- Hallstrom A, Rea TD, Sayre MR, et al. Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: a randomized trial. JAMA 2006;295:2620-8. [PubMed]
- Ong ME, Ornato JP, Edwards DP, et al. Use of an automated, load-distributing band chest compression device for out-of-hospital cardiac arrest resuscitation. JAMA 2006;295:2629-37. [PubMed]
- Wik L, Olsen JA, Persse D, et al. Manual vs. integrated automatic load-distributing band CPR with equal survival after out of hospital cardiac arrest. The randomized CIRC trial. Resuscitation 2014;85:741-8. [PubMed]
- Halperin HR, Guerci AD, Chandra N, et al. Vest inflation without simultaneous ventilation during cardiac arrest in dogs: improved survival from prolonged cardiopulmonary resuscitation. Circulation 1986;74:1407-15. [PubMed]
- Pinto DC, Haden-Pinneri K, Love JC. Manual and automated cardiopulmonary resuscitation (CPR): a comparison of associated injury patterns. J Forensic Sci 2013;58:904-9. [PubMed]
- Wind J, Bekkers SC, van Hooren LJ, et al. Extensive injury after use of a mechanical cardiopulmonary resuscitation device. Am J Emerg Med 2009;27:1017.e1-2.
- Chan PS, Nichol G, Krumholz HM, et al. Hospital variation in time to defibrillation after in-hospital cardiac arrest. Arch Intern Med 2009;169:1265-73. [PubMed]
- Sasson C, Rogers MA, Dahl J, et al. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2010;3:63-81. [PubMed]
- Sanders AB, Ogle M, Ewy GA. Coronary perfusion pressure during cardiopulmonary resuscitation. Am J Emerg Med 1985;3:11-4. [PubMed]
- Ochoa FJ, Ramalle-Gómara E, Lisa V, et al. The effect of rescuer fatigue on the quality of chest compressions. Resuscitation 1998;37:149-52. [PubMed]
- Ikeno F, Kaneda H, Hongo Y, et al. Augmentation of tissue perfusion by a novel compression device increases neurologically intact survival in a porcine model of prolonged cardiac arrest. Resuscitation 2006;68:109-18. [PubMed]
- Brooks SC, Hassan N, Bigham BL, et al. Mechanical versus manual chest compressions for cardiac arrest. Cochrane Database Syst Rev 2014;2:CD007260. [PubMed]
- Cave DM, Gazmuri RJ, Otto CW, et al. Part 7: CPR techniques and devices: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122:S720-8. [PubMed]


