Remote hemodynamic monitoring for ambulatory left ventricular assist device patients
Introduction
Approximately 6 million Americans and 15 million Europeans are living with heart failure (1,2). Guideline directed medical therapy (including angiotensin converting enzyme inhibitors, beta-blockers, and aldosterone antagonists) and devices (implantable cardioverter-defibrillators and cardiac resynchronization therapy) have markedly improved heart failure survival. Despite these treatments, heart failure is associated with significant morbidity and mortality and advanced heart failure persists in a large group of patients. In the United States, it has been estimated that 150,000-250,000 patients suffer with advanced stage heart failure (3). With improving outcomes, growing experience managing the devices, and a greater acceptance of implantation as destination therapy, left ventricular assist devices (LVADs) have become a life-saving option for patients with advanced heart failure (4).
There are over 1 million annual heart failure hospitalizations in the United States and heart failure is associated with the highest rate of hospital readmission of any disease state (1,5). Even with left ventricular output markedly improved after LVAD implant, heart failure related complications can persist due to ineffective LV unloading, arrhythmias, right ventricular (RV) failure, or aortic insufficiency. Hospital readmissions also remain a significant burden for LVAD patients occurring at a rate of 1.5-2.5 per patient year of support, with a higher rate in the first 6 months (6-8). The leading cause of readmissions is gastrointestinal bleeding followed by heart failure and arrhythmia (7).
Due to the complexity and chronicity of heart failure, patient self-management at all stages of the disease is a crucial part of the overall management strategy. However, traditional monitoring approaches for heart failure patients are insensitive and have failed to reduce hospitalization rates or improve quality of life. Recent developments in remote hemodynamic monitoring have been shown to dramatically decrease heart failure hospitalizations and improve quality of life. Remote hemodynamic monitoring can theoretically improve LVAD management by aiding pump speed optimization, medication titration, and timing of transplantation. We will review the current approaches for remote monitoring of heart failure patients and describe how they may be applied to LVAD patients.
Traditional heart failure monitoring
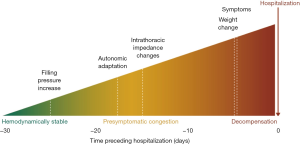
Chronic heart failure management traditionally has focused on noninvasive markers of clinical status. Patients are asked to comply with complex medication regimens, follow diet and fluid restrictions, and actively engage with their care team. In particular, they are instructed to monitor their weight daily, check daily for edema, monitor their symptoms and either adjust their diuretic use according to these parameters or remain in close contact with their provider to determine medication adjustments. While these self-management strategies can decrease rehospitalizations, many patients lack self-care skills and complex instructions can overwhelm resulting in inadequate self-care (9-11). Published compliance with daily weights has been as low as 14-35% (10,11). Symptom monitoring is among the worst performed self-care activities, with compliance rates as low as 9% (10). Even when patients are compliant with self-care instructions, current monitoring strategies are unreliable. Weight gain has poor sensitivity for identifying patients at risk for acute decompensation (Table 1) (12,13). Physical examination findings such as elevated jugular venous pressure, edema, third heart sounds, and rales also all have sensitivities of less than 50% for determining a patient’s hemodynamic status (14). Weight gain, symptoms of congestion, and physical exam findings are often late manifestations of worsening heart failure accounting for their limited impact on reducing hospitalizations and improving heart failure outcomes (Figure 1) (15).
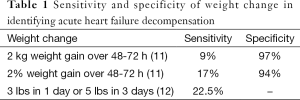
Full table
Telemonitoring strategies, whereby patients are given equipment such as blood pressure monitors and scales to obtain physiologic data at home, have also been attempted to improve heart failure outcomes. Two large multicenter trials suggest that the value of telemonitoring in isolation for heart failure disease management is limited. A large study sponsored by the National Institutes of Health, TELE-HF, enrolled 1,653 patients from 33 sites in the United States (16). Patients had all been recently hospitalized for heart failure and were randomized to usual care or telemonitoring using an automated telephone based system that collected daily information about symptoms and weight. There was no difference between groups in all-cause mortality or heart failure related hospitalizations. Another large randomized controlled trial of telemonitoring, TIM-HF, enrolled 710 patients at 165 sites in Germany to usual care or daily remote monitoring that included blood pressure and weight measurements, electrocardiogram, and medical telephone support (17). After almost two years of follow-up on average, there were no differences between groups in all-cause mortality, cardiovascular death, or heart failure hospitalizations.
Remote hemodynamic monitoring
The failure of noninvasive remote monitoring of heart failure has led to the development of a variety of other hemodynamic approaches to more accurately predict worsening heart failure and reduce hospitalizations. Remote hemodynamic monitoring has the advantage of identifying increases in intracardiac and pulmonary pressures that may precede the development of heart failure symptoms by days to weeks (15). Devices that observe RV pressure, pulmonary artery pressure (PAP), and left atrial pressure have been developed. Recently, a novel system that remotely monitors PAP became the first implantable hemodynamic monitor for heart failure approved by the United States Food and Drug Administration (FDA) (18).
RV pressure monitoring
The RV pressure at the time of pulmonary valve opening reliably estimates PAP (19). This concept was used to develop an implantable RV pressure monitoring system similar to a single-lead pacemaker. This device continuously monitored and recorded estimated pulmonary artery diastolic (ePAD) pressure, heart rate, body temperature, and patient activity. The Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS-HF) trial evaluated this implantable RV monitoring system and was the first randomized controlled trial of remote invasive hemodynamic monitoring in heart failure (20). In COMPASS-HF, 274 New York Heart Association (NYHA) Class III and ambulatory class IV patients were randomized to usual care alone versus usual care guided by the information from the RV monitoring system. The primary outcome was a reduction in the rate of heart failure related events (hospitalizations and emergency or urgent care visits requiring intravenous therapy). There was a non-significant 21% reduction (P=0.33) in primary events in the RV monitoring group. Retrospective analysis showed a 36% reduction (P=0.03) in the relative risk of first heart-failure related hospitalization. Interestingly, COMPASS-HF did confirm that elevated PAP is associated with an increased risk of heart failure events (21). Estimated PAP greater than 25 mmHg was associated with a significantly higher risk of heart failure events when compared to pressures between 10 and 24 mmHg. Unfortunately, clinicians in COMPASS-HF generally failed to adequately target a lower ePAD so the theory that invasive monitoring and lower pressures would significantly reduce hospitalizations remained insufficiently tested.
PAP monitoring
The CardioMEMSTM HF system is a wireless, implantable PAP monitoring system. The system consists of a PAP sensor, external electronics measuring system, and secure website where clinicians can monitor the hemodynamic data (Figure 2). The pulmonary artery sensor is implanted by right heart catheterization via a femoral approach using a special delivery system. The sensor does not have a battery and never needs to be replaced. Patients are instructed to take daily pressure readings from home using the electronics measuring system. Information is transmitted from the measuring system to the secure website where it is immediately available for clinician review.

Remote PAP guided management of heart failure was evaluated in the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial (22,23). All 550 patients in the CHAMPION trial received the CardioMEMS HF System implant. Patients were randomized to a treatment group where clinicians had access to daily PAP measurements versus a control group where clinicians did not have access to daily PAP measurements and used the standard of care only. Patients in the treatment group were managed to protocol specific pressure goals of pulmonary artery systolic pressure of 15 to 35 mmHg, pulmonary artery diastolic pressure of 8 to 20 mmHg, and pulmonary artery mean pressure of 10 to 25 mmHg. The primary endpoint was the rate of heart failure hospitalizations and secondary endpoints included changes in PAP, days alive outside the hospital, and quality of life. Compared to the control group, patients with PAP guided management had a significant 28% reduction in heart failure related hospitalizations at 6 months and 37% reduction in heart failure hospitalizations at 15 months. PAP guided management also resulted in significant reductions in PAP, increased days alive out of the hospital, and improved quality of life. There were no pressure sensor failures and 98.6% freedom from device-related or system-related complications in the CHAMPION trial. These results led to FDA approval of the CardioMEMS HF system for NYHA class III patients on May 28, 2014 (18).
Left atrial pressure monitoring
A system for monitoring left atrial pressure has been developed and consists of an implantable sensor lead coupled with a subcutaneous antenna coil (24,25). The sensor system is implanted using venous access and trans-septal crossing of the interatrial septum. Left atrial pressure, temperature, and intra-cardiac electrocardiogram are monitored. The sensor is powered and interrogated through the skin using a wireless transmitter with high fidelity waveforms (left atrial pressure and intra-cardiac electrocardiogram) captured and available for clinician review through a secure website. Preliminary evaluation of the implanted left atrial pressure sensor demonstrated potentially promising results. The Hemodynamically Guided Home Self-Therapy in Severe Heart Failure Patients (HOMEOSTASIS) trial observed 40 patients with NYHA class III or IV heart failure implanted with a left atrial pressure monitor (26). After an initial 3-month observation period where patients and clinicians were blinded to readings, left atrial pressure measurements and individualized therapy instructions guided medication dosing. The pressure guided and patient self-management treatment was associated with reduced rate of heart failure hospitalizations, lower mean left atrial pressure, increased left ventricular ejection fraction, and improved NYHA class. The self-management approach is similar to diabetes care where patients modify their prescribed insulin dose based on daily objective measures of blood glucose and offers promising opportunities to transform the chronic care of heart failure patients. These findings are being evaluated further in a large prospective randomized trial (27).
Ventricular assist devices and current challenges
LVADs have been shown to greatly improve mortality and quality-of-life in advanced heart failure patients (28), but patients who have undergone implantation with a VAD are still at risk of recurrent heart failure as previously noted. Because of this ongoing risk, a need exists to identify and employ adequate monitoring strategies to treat heart failure after implantation. Some of the traditional noninvasive measures can still be used with VAD patients, such as weight monitoring and evaluation of clinical signs of congestion (e.g., lower extremity edema). There is no evidence, however, that these strategies are any more useful in mechanically supported patients compared to standard heart failure patients; the same lack of sensitivity is assumed to carry over following implantation.
Furthermore, certain physiologic parameters are difficult to assess in the setting of current generation continuous-flow devices. The ability to measure and interpret systemic blood pressures continues to be a challenge for those caring for VAD patients due to the continuous flow produced by contemporary devices. Currently recommended practices suggest the use of Doppler signals to measure peripheral blood pressures, particularly in the absence of a palpable pulse (29). In this setting, Doppler return-to-flow values are suggested to equate to mean arterial pressures (30). Data has been presented, though, to question the accuracy of this recommendation (31). At present, debate still exists whether Doppler-obtained pressures are indicative of a mean arterial pressure or a systolic blood pressure. In addition, the ability to obtain portable Doppler machines to allow patients to check blood pressures at home is severely limited by cost and availability, but has been shown to be feasible (32).
Updated controllers for the current generation of VADs display several pump-specific parameters that can be reported back to care providers to assist in the remote management of patients. Although variances exist based on manufacturer, most devices report the rotational speed of the contained impeller and the instantaneous power consumption of the pump. Axial flow pumps also provide an estimation of pulsatility within the pump. From these parameters, an estimation of blood flow is calculated. It is important to note that the numerical value of flow is not measured and lacks precision based on estimations required for the calculation (33,34). Monitoring trends of these values can assist in the management of these patients (35), but overall they do not have enough sensitivity to detect worsening heart failure or other cardiovascular changes. Rather, they are useful in managing the pump itself and identifying more significant and severe clinical problems.
Newer generation pumps will aim to improve internal accuracy of the assumptions used in calculations to better assist in patient management. One example is the integration of an ultrasonic flow probe in the outflow graft to provide true flow measurements instead of calculated values (36). In one published case series, the use of the integrated flow probe technology along with remote monitoring of additional parameters was shown to be useful in the management of the VAD patient (37). The use of this technology is still undergoing testing and is not yet approved for clinical use.
The ongoing risk of recurrent heart failure after VAD implantation opens the door to innovative strategies for patient management. One approach would be to use remote hemodynamic monitoring in VAD patients. The validity and effectiveness of this strategy has not yet been shown. In theory, trends in PA pressures can predict HF events in mechanically supported patients similar to non-supported HF patients. Unclear at this point, though, is the correlation of specific parameters to worsening heart failure. The presence of a mechanical device within the left side of the heart may reduce the sensitivity of PA diastolic pressures trend as a means to detect volume retention. Additionally, the ability of the CardioMEMS device to accurately detect right-heart failure is unknown. Interest in understanding the relationship is high, and deserves to be the focus of future investigations.
Invasive hemodynamic monitoring theoretically offers other advantages to VAD patients beyond reducing recurrent heart failure admissions. Increases in PAPs that correlate with a rise in serial lactate dehydrogenase (LDH) may more precisely identify device thrombosis, even before LDH reaches currently accepted thresholds or the patient develops significant symptoms (38). PAP monitoring may also allow for more accurate pump speed modifications to determine effective unloading of the left ventricle. In particular, patients with secondary pulmonary hypertension that precludes primary cardiac transplantation may be able to be managed more effectively without repeated right heart catheterizations to monitor PAP. This would eliminate frequent interruptions in anticoagulation and the potential associated complications. Finally, in addition to recording PAP, the CardioMEMS system monitors heart rate allowing for earlier detection of tachy-arrhythmias that may compromise VAD function.
Moving forward, the challenge will be to create “smart” pumps that integrate biofeedback of physiologic parameters. Utilization of feedback data may allow pumps to adjust themselves to match the needs of the patient; much the way modern pacemaker technology allows self-adjustment. Although pacemaker feedback mechanisms have become quite sophisticated and can integrate a variety of parameters such as respiratory rate, movement, and oxygen demand, direct carry over to VAD technology is not yet possible. The need to follow right-ventricular function, filling pressures, and pump flow are essential to creating a “smart” pump that can react and self-adjust. Moreover, the relative importance of these parameters in relation to each other still needs further understanding. For example, the significance of heart rate on VAD function is not well understood. If important, a “smart pump” may need to communicate with a pacemaker or have integrated heart rate control mechanisms. Integration of these parameters has been explored in simulations (39), but has not yet been implemented into the manufacturing process. Although elusive, the realization of these features into commercially available products continues to be necessary and will shape the future of the field.
Acknowledgements
None.
Footnote
Conflicts of Interest: B Lampert—St. Jude Medical: consultant; S Emani—Thoratec: consultant, grant funding; St. Jude Medical: consultant, grant funding.
References
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28-e292. [PubMed]
- Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008;29:2388-442. [PubMed]
- Miller LW, Guglin M. Patient selection for ventricular assist devices: a moving target. J Am Coll Cardiol 2013;61:1209-21. [PubMed]
- Jorde UP, Kushwaha SS, Tatooles AJ, et al. Results of the destination therapy post-food and drug administration approval study with a continuous flow left ventricular assist device: a prospective study using the INTERMACS registry (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2014;63:1751-7. [PubMed]
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009;360:1418-28. [PubMed]
- Forest SJ, Bello R, Friedmann P, et al. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg 2013;95:1276-81. [PubMed]
- Hasin T, Marmor Y, Kremers W, et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol 2013;61:153-63. [PubMed]
- Smedira NG, Hoercher KJ, Lima B, et al. Unplanned hospital readmissions after HeartMate II implantation: frequency, risk factors, and impact on resource use and survival. JACC Heart Fail 2013;1:31-9. [PubMed]
- Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord 2006;6:43. [PubMed]
- Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J 2005;150:984. [PubMed]
- van der Wal MH, Jaarsma T, Moser DK, et al. Compliance in heart failure patients: the importance of knowledge and beliefs. Eur Heart J 2006;27:434-40. [PubMed]
- Abraham WT, Compton S, Haas G, et al. Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: results of the Fluid Accumulation Status Trial (FAST). Congest Heart Fail 2011;17:51-5. [PubMed]
- Lewin J, Ledwidge M, O'Loughlin C, et al. Clinical deterioration in established heart failure: what is the value of BNP and weight gain in aiding diagnosis? Eur J Heart Fail 2005;7:953-7. [PubMed]
- Capomolla S, Ceresa M, Pinna G, et al. Echo-Doppler and clinical evaluations to define hemodynamic profile in patients with chronic heart failure: accuracy and influence on therapeutic management. Eur J Heart Fail 2005;7:624-30. [PubMed]
- Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep 2009;6:287-92. [PubMed]
- Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med 2010;363:2301-9. [PubMed]
- Koehler F, Winkler S, Schieber M, et al. Telemedical Interventional Monitoring in Heart Failure (TIM-HF), a randomized, controlled intervention trial investigating the impact of telemedicine on mortality in ambulatory patients with heart failure: study design. Eur J Heart Fail 2010;12:1354-62. [PubMed]
- U.S. Food and Drug Administration Center for Devices and Radiological Health. CardioMEMS(TM) HF System approval letter. 2014. Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf10/P100045a.pdf. Accessed June 30, 2015.
- Reynolds DW, Bartelt N, Taepke R, et al. Measurement of pulmonary artery diastolic pressure from the right ventricle. J Am Coll Cardiol 1995;25:1176-82. [PubMed]
- Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol 2008;51:1073-9. [PubMed]
- Stevenson LW, Zile M, Bennett TD, et al. Chronic ambulatory intracardiac pressures and future heart failure events. Circ Heart Fail 2010;3:580-7. [PubMed]
- Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658-66. [PubMed]
- Adamson PB, Abraham WT, Aaron M, et al. CHAMPION trial rationale and design: the long-term safety and clinical efficacy of a wireless pulmonary artery pressure monitoring system. J Card Fail 2011;17:3-10. [PubMed]
- Ritzema J, Melton IC, Richards AM, et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: initial experience with a new permanent implantable device. Circulation 2007;116:2952-9. [PubMed]
- Troughton RW, Ritzema J, Eigler NL, et al. Direct left atrial pressure monitoring in severe heart failure: long-term sensor performance. J Cardiovasc Transl Res 2011;4:3-13. [PubMed]
- Ritzema J, Troughton R, Melton I, et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation 2010;121:1086-95. [PubMed]
- Maurer MS, Adamson PB, Costanzo MR, et al. Rationale and Design of the Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy Study (LAPTOP-HF). J Card Fail 2015;21:479-88. [PubMed]
- Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol 2010;55:1826-34. [PubMed]
- Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant 2010;29:S1-39. [PubMed]
- Bennett MK, Roberts CA, Dordunoo D, et al. Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant 2010;29:593-4. [PubMed]
- Lanier GM, Orlanes K, Hayashi Y, et al. Validity and reliability of a novel slow cuff-deflation system for noninvasive blood pressure monitoring in patients with continuous-flow left ventricular assist device. Circ Heart Fail 2013;6:1005-12. [PubMed]
- Lampert BC, Eckert C, Weaver S, et al. Blood pressure control in continuous flow left ventricular assist devices: efficacy and impact on adverse events. Ann Thorac Surg 2014;97:139-46. [PubMed]
- HeartMate II LVAS Operating Manual. Available online: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4333b2-18-%209_2%20HM%20II%20Operating%20Manual.pdf
- HeartWare Ventricular Assist System Instructions for use. Available online: http://www.heartware.com/sites/default/files/uploads/docs/ifu00001_rev_15.pdf
- Slaughter MS, Bartoli CR, Sobieski MA, et al. Intraoperative evaluation of the HeartMate II flow estimator. J Heart Lung Transplant 2009;28:39-43. [PubMed]
- FlowAccurate Diagnostics. Available online: http://reliantheart.com/heartassist5/new-heart-assist-5/flowaccurate-diagnostics/. Accessed September 7, 2015 2015.
- Pektok E, Demirozu ZT, Arat N, et al. Remote monitoring of left ventricular assist device parameters after HeartAssist-5 implantation. Artif Organs 2013;37:820-5. [PubMed]
- Shah P, Mehta VM, Cowger JA, et al. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. J Heart Lung Transplant 2014;33:102-4. [PubMed]
- Ferreira A, Boston JR, Antaki JF. A control system for rotary blood pumps based on suction detection. IEEE Trans Biomed Eng 2009;56:656-65. [PubMed]


