Design and implementation of an enhanced recovery program in thoracic surgery
Introduction
Despite significant improvements in perioperative care, major surgery is still associated with a number of major complications, such as respiratory failure, cardiovascular instability, acute renal impairment, infection and neurological events (1-3). After thoracic surgery, acute lung injury, persistent air leak, and chronic pain are amongst the commonest complications (4-6). In a variety of video-assisted thoracoscopy (VATS) procedures, the overall complications rate has been shown to be between 3.7% and 20% (7-10). The incidence of minor and major complications after lobectomy via thoracotomy in the first 30 days has been reported to be 48.5% and 35.7% respectively (10). Mortality has also been shown to vary, from 1.4% to 2% for anatomical lung resection by VATS (6,11), to 3.7% in a historical cohort of all types of lung resections (12).
In the late 1990s, Kehlet et al. (13) studied the modulation of the perioperative stress, and the underneath mechanism related to metabolic and immunological response to surgical stimulation. Implementation of better pain control and the introduction of minimally invasive surgical techniques were thought to decrease the stress response and surgical stimulation, respectively, and eventually reduce perioperative complications and length of hospital stay (14,15). Later, Kehlet et al. designed and implemented a care package for patients undergoing elective colorectal surgery, whose aim was to decrease perioperative complications and length of stay (14). The concept rapidly spread to a variety of surgical specialties, including orthopedic, gynaecological, liver, ear nose and throat, cardiac, and more recently thoracic surgery. Our institution is one of the pioneers in re-engineering the evidence based perioperative pathway for thoracic surgery patients, in which we have incorporated the core principles of perioperative optimization and enhanced recovery (ER).
In this article, we have reviewed the recent literature about ER in thoracic surgery, and also describe our institutional experience in the design, implementation and monitoring of changes to the perioperative thoracic pathway.
Methods
We searched the MEDLINE and EMBASE databases. The following search terms were used: “enhanced recovery”, “fast track surgery”, “thoracic surgery”, “prehabilitation”, “pain thoracotomy”, “inflammatory response”, “length of stay”, “complications in thoracic surgery”, “VATS surgery”. Studies included in the review were limited to those in the English language. Studies found were analysed by the authors and based on their relevance they were included in the review.
Overview of ER programs
When ER is applied to colorectal surgery, it has been shown to reduce postoperative complications and length of stay (16). Two recent meta-analyses comparing ER with conventional care have shown a reduction in both, with a very similar readmission rate (17,18). However, variation between institutions may make it difficult to assess the real impact of the interventions (19). Moreover, the degree of evidence and hence the applicability of different interventions varies between specialties, and colorectal surgery is the area in which ER care has been most studied (20,21).
Generally, ER interventions are divided into the preoperative, intraoperative and postoperative phases. Preoperative interventions aim to ensure that the patient is in the best possible condition for surgery; the preoperative protocol should include nutritional assessment and treatment, anaemia correction and smoking cessation advice. Likewise, optimization of underlying pathologies such as diabetes mellitus, systemic hypertension, and chronic obstructive pulmonary disease is performed. Physical activity promotion and educational programs that provide information about surgery and the ER pathway are an essential part of the preoperative strategies, as well as a plan for date of admission and discharge.
In the intraoperative phase, an optimal regional analgesic technique is required, as well as perioperative hypothermia prevention, antibiotic prophylaxis, avoidance of fluid overload, minimising surgical drains, use of short acting anaesthetic agents, and minimally invasive surgery where possible.
Finally, in the postoperative period, an appropriate level of postoperative care is required depending on comorbidities (i.e., high dependency, intensive care or general ward), aggressive pain management, early mobilization, early oral intake, and prophylaxis of nausea and vomiting are the main targets.
In addition to these specific measures, the ER program relies on standardization of the care pathway, in which the multidisciplinary team work together and a cultural drive for change is the cornerstone of the program. Different professionals such as general practitioners, anaesthetists, surgeons, physiotherapists, specialist nurses, and dietitians should all work altogether in a coordinated manner to deliver the best clinical care and obtain the best possible outcome.
ER in thoracic surgery
The development of an ER program in thoracic surgery has received less attention than other surgical areas; nonetheless, its introduction has shown to decrease postoperative complications (21,22) and reduce length of stay (23). In a prospective randomised controlled trial that compared fast-track lung resection with conventional care, the fast-track group had fewer complications (6.6%) than the control group (35%) (24,25). Since the intervention was tested contemporaneously in a single institution, not surprisingly, length of stay in hospital did not differ between the two groups (21,22). This highlights the importance of a whole cultural change within an institution where all interventions are coordinated and embraced by all those who deliver perioperative care.
Preventza et al. (23) in a retrospective review, found that after VATS wedge resection, only 8% of patients stayed more than 5 days in hospital, 22% patients stayed 2 days and 70% of patients were discharged within a day. Das-Neves-Pereira et al. (24) published a retrospective study including 109 patients undergoing lobectomy via thoracotomy in an ER program, and reported fewer complications in the group of patients who followed the fast-track protocol in comparison with the group who did not followed the program.
Papworth ER program experience
The Papworth ER pathway incorporates most of the main aspects of a conventional ER program, such as universal preoperative anaesthetic assessment, optimization of comorbidities and patient education (Table 1). All cases are discussed by a multidisciplinary team, which includes radiologists, oncologists, chest physicians and thoracic surgeons. Preoperative optimization starts early in the pathway, when patients are considered for surgery. Nonetheless, a final assessment is provided in a “single stop” clinic where patients are consented for surgery as well as receive a preoperative anaesthetic assessment. A special focus is put on working in partnership with patients regarding their own care. An estimated day of discharge is discussed and a diary with expected postoperative progress is handed to all patients.
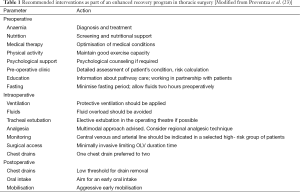
Full table
In principle, all patients are eligible for day of surgery admission unless there is a clinical contraindication (i.e., patients needs dialysis before surgery) or social support limitation (i.e., patient lives more than three hours away from hospital or has not enough familiar support at home). Clear fluid intake up to 2 h before surgery is allowed (25) and patients are transported to theatre in a wheelchair to maintain mobility as much as possible.
Intraoperatively, invasive arterial monitoring and peripheral venous access are used. We avoid routine insertion of urinary catheters and central venous lines (Table 1). The administration of short acting anaesthetic drugs, together with multimodal analgesia with non-steroidal anti-inflammatory drugs (unless contra-indicated), paracetamol, opiates and a surgically-inserted paravertebral catheter allows good pain relief with minimal respiratory depression. Therefore, early tracheal extubation in the operating theatre is facilitated. Aggressive prevention and treatment of inadvertent hypothermia are part of the standard of practice.
In our centre, when appropriate, the preference is to insert one chest drain, as opposed to several. No differences in amount of drainage and length of stay have been demonstrated comparing 2 vs. 1 chest drain, however, one chest drain confers less postoperative pain and enables earlier mobilization (25). After immediate tracheal extubation in the operating theatre, patients are transferred to the recovery area where they are closely monitored and any immediate postoperative complications are actively managed (Table 1). Optimal pain control is paramount. If patients experience moderate to severe pain on arrival in the recovery ward, an immediate (within 10 min of arrival) extra dose of intravenous opiate is administered, and/or an additional regional analgesic technique is performed (i.e., intercostal block, paravertebral block or epidural block), by an experienced anaesthetist.
Our ER program includes early mobilization and early chest drain removal. A higher threshold for drain removal (<450 mL non-chylous, non-hemorrhagic drainage post lung resection) has been shown to be safe (26); it reduces pain and facilitates effective mobilization. Acute kidney injury (AKI) is not uncommon after thoracic surgery, with an incidence of up to 5.9% (27,28). Intraoperative administration of hydroxyethyl starch is a proposed risk factor for AKI (29) in thoracic surgery, and therefore we never use this in our institution.
Optimal fluid therapy management is a controversial topic. The description of the relationship between acute lung injury and liberal fluid administration led to the adoption of restrictive fluid management (30). Nevertheless, no goal directed therapy to guide this restrictive fluid approach has been validated for thoracic surgery (31). In our clinical practice, intraoperative fluid administration is guided by clinical criteria. Insensible losses throughout surgery are replaced by the administration of Hartmann’s solution at a rate of 1–2 mL/kg/h. If the clinical condition requires volume expansion, we use small boluses of Gelofusine®. As part of our fluid regimen, we administer Hartmann’s Solution at a rate of 1–1.5 mL/kg/h for 12 h after surgery, and stop when adequate oral intake is confirmed.
Analgesic technique: from epidural to paravertebral
Initially, the first ER protocols defined epidural analgesia as an essential part of the bundle of care, and it has been the gold standard technique for pain control after major surgery for some time. However, its adverse effects, such as urinary retention, low blood pressure and muscular weakness, makes epidural analgesia less attractive as part of an ER program, especially in elderly patients (32,33). In addition, an increasing number of patients present with dual antiplatelet therapy, renal failure and oral anticoagulation, which may increase the potential risk of epidural bleeding and its devastating complications (34).
Paravertebral analgesia provides a unilateral block of somatic and sympathetic nerves that lie in the paravertebral space, and is particularly useful in unilateral chest and abdominal procedures (35,36). It is a block that can be performed using landmarks technique with loss of resistance (37), can be guided by a nerve stimulator (38), or use of ultrasound (39). Due to the variation in efficacy with the above-mentioned techniques, a paravertebral catheter inserted during surgery under direct vision has gained popularity in recent years (40). In 2006, Davies et al. (33) published a systematic review comparing epidural with paravertebral block in thoracic surgery, and reported similar pain scores. Patients in the paravertebral group had fewer failed blocks and fewer side effects like urinary retention, hypotension and nausea. The authors concluded that paravertebral blocks could be used effectively in thoracic surgery.
At our institution, the surgically inserted paravertebral catheter with a bolus dose of local anaesthetic followed by a continuous infusion of local anaesthetic is the technique of choice. Due to the latency of the paravertebral block, the catheter is usually inserted at least 30 to 40 min before the patient is woken up. There are no available studies comparing surgically-inserted with percutaneous paravertebral catheters. However, the percutaneous approach ensures administration of local anaesthetic thorough the procedure with a pre-emptive analgesic effect. This analgesic administration before the surgical stimulation occurs has been demonstrated to have an effect on acute pain but not in preventing chronic pain (41,42).
Although a regional technique in general and paravertebral block in particular are an essential part of our perioperative pain control strategy, multimodal analgesia is paramount in thoracic surgery. At our institution, all patients receive a short acting opiate, cyclo-oxygenase-2 non-steroidal anti-inflammatory drug, paracetamol and morphine patient controlled analgesia. The role of ketamine in thoracic surgery for acute pain and prevention of chronic pain is controversial. In a recent meta-analysis (43), the authors concluded that the addition of ketamine to PCA morphine provides better pain control compared with morphine-PCA alone. However, two recent trials have shown that in patients who received epidural and PCA analgesia, adding intravenous or epidural ketamine did not provide better pain relief, probably due to the optimized analgesia technique (44,45). The benefit of ketamine on paravertebral block analgesia for thoracic surgery has not been explored.
The use of gabapentin in a single preoperative oral dose does not reduce pain scores or morphine consumption (46). However, preoperative and postoperative administration for 2 days provides better pain relief compared to PCA-morphine alone. There are no studies testing gabapentin combined with regional analgesia techniques in thoracic surgery.
Acute lung injury in minimally invasive thoracic surgery
One-lung ventilation (OLV) is necessary for most thoracic surgical procedures, but is associated with a great many of the respiratory complications after thoracic surgery, including acute lung injury, which has an incidence between 4% and 15%, depending on the type of lung resection (11,47). Furthermore, patients who develop postoperative acute lung injury or acute respiratory distress syndrome have an increased risk of death after thoracic surgery (48-50). During OLV, both lungs are prone to tissue damage; the ventilated lung is prone to high non-physiologic tidal volumes, and re-expansion of the collapsed lung may be followed by a reperfusion injury response. This inflammatory response is triggered by cytokine release that can initiate further damage locally and also to the contralateral lung. Modulation of this response has been achieved by reducing OLV time, applying continuous positive airway pressure (CPAP) to the collapsed lung (51), and by the use of volatile anaesthetics (52). The use of sevoflurane and desflurane compared with total intravenous anaesthesia with propofol has shown to decrease the release of pro-inflammatory mediators (4), but the clinical correlation with outcomes such as acute respiratory distress syndrome has only been demonstrated in a single randomized controlled trial (53).
The use of VATS surgery may reduce the stress response, but equally can be associated with longer OLV periods, depending on operator experience and surgical difficulty. In the context of perioperative ER interventions, the benefits of an open thoracotomy with an experienced surgeon and short OLV has to be weighed against a relatively inexperienced VATS surgeon and prolonged OLV time.
Although VATS surgery appears to be the preferred surgical approach as part of the ER program for patients undergoing lung resection, some studies have shown similar results with an open thoracotomy approach. Cerfolio et al. (5) presented a series of 500 single-surgeon consecutive resections including lobectomy and pneumonectomy via open thoracotomy, but maintaining all other perioperative aspects of ER pathway. The median day of discharge was day four postoperatively, with a morbidity of 21% and mortality of 2%. The results point out that the whole ER care package (high-volume centre, experienced surgeon, immediate extubation, good analgesic technique) could potentially be more important than the surgical technique by itself.
In order to decrease the complications after thoracic surgery, prophylactic high-flow nasal oxygen therapy compared with standard oxygen has been recently tested in a randomized controlled trial (54). It delivers a flow-dependent positive airway-pressure and may offer a better tolerability profile than CPAP. High-flow nasal oxygen did not improve functional capacity measured by the 6-minute test walk, but it did significantly reduce length of stay in hospital from 4 to 2.5 days in patients who received high-flow nasal oxygen immediately after tracheal extubation for 24 h.
In our centre, hospital length of stay was reduced by about 4 days after the introduction of our ER program, despite the fact that in the first year of implementation, the majority of the patient still underwent open thoracotomy (unpublished data). When minimally invasive VATS became routine surgical practice, there was a further marginal reduction in length of stay. Therefore, although it is difficult to assess the magnitude of the impact of VATS on an ER program, its introduction appears to be beneficial.
In summary, OLV should always be kept as short as possible aimed in order to decrease respiratory complications. Despite the surgical access method chosen, perioperative care should be part of a multidisciplinary ER program in order to obtain the best possible outcomes.
Prehabilitation in ER
Pre-rehabilitation is a novel concept that that is based on promoting preoperative physical activity in order to enhance tolerance to surgery and facilitate postoperative recovery (55,56). In 2005, a systematic review was published looking at the effect of a physical activity pre-rehabilitation program on patients undergoing surgery, which showed limited impact (55). Later on, an integral prehabilitation program performed in the preoperative period was compared with a rehabilitation program, which was only applied postoperatively. The interventions in both groups were based on aerobic exercise, nutritional counseling with protein supplementation, and relaxation exercises. The authors found that the prehabilitation group had significant improvements in postoperative functional exercise capacity measured by 6-minute walk test at 4 and 8 weeks after the procedure (57). Physical and cognitive prehabilitation in animals has also been shown to attenuate neuro-inflammation and decrease the incidence of postoperative cognitive dysfunction (58). Further studies exploring the impact of prehabilitation in thoracic surgery are needed, in order to establish the benefit of including prehabilitation in ER protocols.
Patient suitability for ER
One of the aims of the preoperative anaesthetic assessment is to identify high-risk patients, with the objective of optimizing them before surgery, and also for planning postoperative care requirements. Poor preoperative functional capacity (VO2max <15 mL/kg/min) or poor preoperative lung function (FEV1 or TLCO <40%) are recognized risk factors for complications following thoracic surgery (59). Moreover, the Thoracoscore can be used to predict mortality using nine variables (60) (Table 2). This can help identify patients who are at risk of suffering perioperative complications. However, it is not effective enough to identify patients who are “off ER pathway” or establish to what degree perioperative care should be modified in such patients.
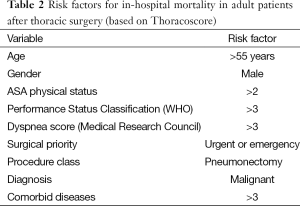
Full table
Most patients included in a thoracic surgical ER program will be scheduled for lung cancer resection. Addiction to smoking will be frequently seen, as well as ischaemic heart disease, peripheral vascular disease and some degree of renal impairment. Preoperative comorbidity, along with technical intraoperative issues, has been shown to increase the risk of reoperation after pulmonary resection surgery, increasing mortality and hospital length of stay (61). Alcohol intake should be recorded in order to start preventive treatment of alcohol withdrawal. The presence of preoperative chronic pain should alert the clinician to the necessity of a tailored and intensive pain control regimen.
Some of the ER measures are universal and potentially applicable to all patients, such as immediate tracheal extubation, optimal pain control, early ambulation and oral intake. However, some interventions, which may not be considered to be part of ER, may be required in patients with advanced chronic conditions. Some high-risk patients may need more invasive intraoperative monitoring, elective urinary catheter insertion before surgery, or postoperative intensive care unit admission. Identification of vulnerable patients may facilitate the early recognition and treatment of postoperative complications.
Whether this group of patients, due to the high risk of complications, should be managed more aggressively or be included in an ER program is controversial and varies from one centre to another. In our centre, a modified ER program is applied to high-risk patients such as those with severe cardiac conditions (i.e., severe chronic angina, poor left ventricular function), advanced chronic kidney disease or severe vascular disease. Some of the above-mentioned patients are managed with more invasive monitoring and urinary catheterization. Other high risk patients such as those with poor functional respiratory function (FEV1 <40%) or undergoing extended lung resection such as bilobectomy or pneumonectomy, do not qualify a priori as a reason to alter the whole ER pathway. Nonetheless, the intraoperative team, recovery and thoracic ward have to have a robust system in place to detect early signs of deterioration that may require escalation of care where needed. It still remains to be established which criteria can accurately predict which patient would benefit from full ER program and which patients require special care. In our centre, the decision to alter the pathway is made based on a case-by case basis, integrating patient comorbidities, postoperative predicted respiratory function and surgical difficulty.
Conclusions
Despite the limited evidence and inter-institution variability, implementation of thoracic surgery ER programs seems to reduce hospital stay and possibly reduce postoperative complications. In our experience, successful ER implementation requires institutional support, multidisciplinary involvement and proper education and training for all staff involved in the care of these patients. Delivering highly standardised care is paramount, as well as rapidly identifying “off pathway” patients. Further evidence is needed to quantify the impact of single interventions and the ER bundle of care as a whole.
Acknowledgements
None.
Footnote
Conflicts of Interest: AA Klein has received an educational grant, honoraria and travel expenses from Fisher and Paykel for the study of high-flow nasal oxygen. No other conflicts of interest declared.
References
- Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med 2004;32:916-21. [PubMed]
- Story DA. Postoperative complications in elderly patients and their significance for long-term prognosis. Curr Opin Anaesthesiol 2008;21:375-9. [PubMed]
- McNicol L, Story DA, Leslie K, et al. Postoperative complications and mortality in older patients having non-cardiac surgery at three Melbourne teaching hospitals. Med J Aust 2007;186:447-52. [PubMed]
- Lohser J, Slinger P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg 2015;121:302-18. [PubMed]
- Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. [PubMed]
- Imperatori A, Rotolo N, Gatti M, et al. Peri-operative complications of video-assisted thoracoscopic surgery (VATS). Int J Surg 2008;6 Suppl 1:S78-81. [PubMed]
- Krasna MJ, Deshmukh S, McLaughlin JS. Complications of thoracoscopy. Ann Thorac Surg 1996;61:1066-9. [PubMed]
- Yim AP, Liu HP. Complications and failures of video-assisted thoracic surgery: experience from two centers in Asia. Ann Thorac Surg 1996;61:538-41. [PubMed]
- Jancovici R, Lang-Lazdunski L, Pons F, et al. Complications of video-assisted thoracic surgery: a five-year experience. Ann Thorac Surg 1996;61:533-7. [PubMed]
- Laursen LØ, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2015. [Epub ahead of print]. [PubMed]
- Decaluwe H, Petersen RH, Hansen H, et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98; discussion 599. [PubMed]
- Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7. [PubMed]
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [PubMed]
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630-41. [PubMed]
- Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189-98. [PubMed]
- Kehlet H. Fast-track colonic surgery: status and perspectives. Recent Results Cancer Res 2005;165:8-13. [PubMed]
- Spanjersberg WR, Reurings J, Keus F, et al. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 2011.CD007635. [PubMed]
- Varadhan KK, Neal KR, Dejong CH, et al. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 2010;29:434-40. [PubMed]
- Ahmed J, Khan S, Lim M, et al. Enhanced recovery after surgery protocols - compliance and variations in practice during routine colorectal surgery. Colorectal Dis 2012;14:1045-51. [PubMed]
- Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009;144:961-9. [PubMed]
- Muehling BM, Halter GL, Schelzig H, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. [PubMed]
- Jones NL, Edmonds L, Ghosh S, et al. A review of enhanced recovery for thoracic anaesthesia and surgery. Anaesthesia 2013;68:179-89. [PubMed]
- Preventza O, Hui HZ, Hramiec J. Fast track video-assisted thoracic surgery. Am Surg 2002;68:309-11. [PubMed]
- Das-Neves-Pereira JC, Bagan P, Coimbra-Israel AP, et al. Fast-track rehabilitation for lung cancer lobectomy: a five-year experience. Eur J Cardiothorac Surg 2009;36:383-91; discussion 391-2. [PubMed]
- Scott MJ, Fawcett WJ. Oral carbohydrate preload drink for major surgery - the first steps from famine to feast. Anaesthesia 2014;69:1308-13. [PubMed]
- Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg 2008;135:269-73. [PubMed]
- Ishikawa S, Griesdale DE, Lohser J. Acute kidney injury after lung resection surgery: incidence and perioperative risk factors. Anesth Analg 2012;114:1256-62. [PubMed]
- Licker M, Cartier V, Robert J, et al. Risk factors of acute kidney injury according to RIFLE criteria after lung cancer surgery. Ann Thorac Surg 2011;91:844-50. [PubMed]
- Ahn HJ, Kim JA, Lee AR, et al. The Risk of Acute Kidney Injury from Fluid Restriction and Hydroxyethyl Starch in Thoracic Surgery. Anesth Analg 2016;122:186-93. [PubMed]
- Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007;84:1085-91; discussion 1091. [PubMed]
- Minto G, Struthers R. Stroke volume optimisation: is the fairy tale over? Anaesthesia 2014;69:291-6. [PubMed]
- Horlocker TT. Regional anaesthesia in the patient receiving antithrombotic and antiplatelet therapy. Br J Anaesth 2011;107 Suppl 1:i96-106. [PubMed]
- Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418-26. [PubMed]
- Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026-40. [PubMed]
- Karmakar MK. Thoracic paravertebral block. Anesthesiology 2001;95:771-80. [PubMed]
- Shelley B, Macfie A. Where now for thoracic paravertebral blockade? Anaesthesia 2012;67:1317-20. [PubMed]
- Eason MJ, Wyatt R. Paravertebral thoracic block-a reappraisal. Anaesthesia 1979;34:638-42. [PubMed]
- Lang SA. The use of a nerve stimulator for thoracic paravertebral block. Anesthesiology. 2002;97:521; author reply 521-2. [PubMed]
- Krediet AC, Moayeri N, van Geffen GJ, et al. Different Approaches to Ultrasound-guided Thoracic Paravertebral Block: An Illustrated Review. Anesthesiology 2015;123:459-74. [PubMed]
- Berrisford RG, Sabanathan SS. Direct access to the paravertebral space at thoracotomy. Ann Thorac Surg 1990;49:854. [PubMed]
- Pogatzki-Zahn EM, Zahn PK. From preemptive to preventive analgesia. Curr Opin Anaesthesiol 2006;19:551-5. [PubMed]
- Katz J, McCartney CJ. Current status of preemptive analgesia. Curr Opin Anaesthesiol 2002;15:435-41. [PubMed]
- Mathews TJ, Churchhouse AM, Housden T, et al. Does adding ketamine to morphine patient-controlled analgesia safely improve post-thoracotomy pain? Interact Cardiovasc Thorac Surg 2012;14:194-9. [PubMed]
- Joseph C, Gaillat F, Duponq R, et al. Is there any benefit to adding intravenous ketamine to patient-controlled epidural analgesia after thoracic surgery? A randomized double-blind study. Eur J Cardiothorac Surg 2012;42:e58-65. [PubMed]
- Tena B, Gomar C, Rios J. Perioperative epidural or intravenous ketamine does not improve the effectiveness of thoracic epidural analgesia for acute and chronic pain after thoracotomy. Clin J Pain 2014;30:490-500. [PubMed]
- Zakkar M, Frazer S, Hunt I. Is there a role for gabapentin in preventing or treating pain following thoracic surgery? Interact Cardiovasc Thorac Surg 2013;17:716-9. [PubMed]
- Lohser J. Evidence-based management of one-lung ventilation. Anesthesiol Clin 2008;26:241-72. v. [PubMed]
- Licker M, Fauconnet P, Villiger Y, et al. Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol 2009;22:61-7. [PubMed]
- Fernández-Pérez ER, Sprung J, Afessa B, et al. Intraoperative ventilator settings and acute lung injury after elective surgery: a nested case control study. Thorax 2009;64:121-7. [PubMed]
- Iglesias M, Martinez E, Badia JR, et al. Extrapulmonary ventilation for unresponsive severe acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2008;85:237-44; discussion 244. [PubMed]
- Verhage RJ, Boone J, Rijkers GT, et al. Reduced local immune response with continuous positive airway pressure during one-lung ventilation for oesophagectomy. Br J Anaesth 2014;112:920-8. [PubMed]
- Schilling T, Kozian A, Senturk M, et al. Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology 2011;115:65-74. [PubMed]
- De Conno E, Steurer MP, Wittlinger M, et al. Anesthetic-induced improvement of the inflammatory response to one-lung ventilation. Anesthesiology 2009;110:1316-26. [PubMed]
- Ansari BM, Hogan MP, Collier TJ, et al. A Randomized Controlled Trial of High-Flow Nasal Oxygen (Optiflow) as Part of an Enhanced Recovery Program After Lung Resection Surgery. Ann Thorac Surg 2016;101:459-64. [PubMed]
- Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care 2005;8:23-32. [PubMed]
- Durrand JW, Batterham AM, Danjoux GR. Pre-habilitation. I: aggregation of marginal gains. Anaesthesia 2014;69:403-6. [PubMed]
- Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014;121:937-47. [PubMed]
- Kawano T, Eguchi S, Iwata H, et al. Impact of Preoperative Environmental Enrichment on Prevention of Development of Cognitive Impairment following Abdominal Surgery in a Rat Model. Anesthesiology 2015;123:160-70. [PubMed]
- Wright CD, Gaissert HA, Grab JD, et al. Predictors of prolonged length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk-adjustment model. Ann Thorac Surg 2008;85:1857-65; discussion 1865.
- Falcoz PE, Conti M, Brouchet L, et al. The Thoracic Surgery Scoring System (Thoracoscore): risk model for in-hospital death in 15,183 patients requiring thoracic surgery. J Thorac Cardiovasc Surg 2007;133:325-32. [PubMed]
- Yang Y, Gao W, Zhao H, et al. Risk factors and consequences of perioperative reoperation in patients undergoing pulmonary resection surgery. Surgery 2016;159:591-601. [PubMed]


