Impacts of anemia on 3-year ischemic events in patients undergoing percutaneous coronary intervention: a propensity-matched study
Introduction
Anemia is a common health problem (1), and its prevalence rises with advancing age (2). Previous studies showed that anemia was associated with high mortality, and significantly reduced the life quality of patients in a variety of settings (2-5). Reduction in the hemoglobin (Hb) concentration may compromise oxygen supply to infarcted or ischemic myocardium, which may promote arrhythmias, worsen hypotension, increase infarct size and increase the risk for ischemic events (6-8). Studies have suggested that anemia was an independent risk factor for cardiovascular disease (CVD) in patients with (9-13) or without (14) CVD. Recovery of anemia can improve the outcomes of patients with CVD (15-17).
For patients who underwent percutaneous coronary intervention (PCI), anemia is associated with higher incidence of adverse events (18-22), including the incidence of ischemic events (23). Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Risk Score have identified anemia as a predictor for mortality in patients after primary PCI (24). Anemic patients have more risk factors than nonanemic patients, such as older age (25,26), renal disease (25-27), other chronic diseases (26). These risk factors may have interaction impacts on the outcome in anemic patients. However, most of previous studies didn’t do propensity matching to minimize the influence of other confounders on outcome. Therefore, although these studies have suggested that anemia was associated with the outcome of patients undergoing PCI, whether anemia is an independent predictor of outcome in patients undergoing PCI remains unknown. What’s more, studies focused on the impacts of anemia on long-term outcome, especially long-term ischemic events or mortality, in the patients undergoing PCI is still limited. Ischemic events, such as myocardial infarction, ischemic stroke and revascularization have significant influence on the life quality in patients undergoing PCI.
Thus, the purpose of this study is to (I) identify whether anemia is an independent predictor for 3-year ischemic events, all-cause mortality and major adverse cardiac events (MACE); (II) explore the incidence of 3-year ischemic events in anemic patients and nonanemic patients who underwent PCI.
Methods
Study population
Patients who underwent PCI from July 2008 to November 2012 in General Hospital of Shenyang Military Region were included in this study. Baseline characteristics, cardiac history, risk factors, medications, angiographic and procedural data were prospectively obtained and recorded. The data of follow-up period were collected by telephone interview in 1, 6, 12, 18, 24, and 36 months after PCI.
Clinical outcomes and definitions
The main outcome of this study is 3-year ischemic events after PCI, the secondary outcome of this study is 3-year mortality and MACE after PCI. Anemia was defined as an Hb level <11.0 g/dL for women or <12.0 g/dL for men. Ischemic events were defined as a composite of myocardial infarction, ischemic stroke, revascularization. MACE was defined as a composite of all-cause mortality, non-fatal myocardial infarction, target vessel revascularization (TVR). History of cardiovascular and cerebrovascular diseases was defined as history of myocardial infarction, ischemic stroke, and interventional operation. Complication of PCI included acute vascular occlusion, plaque prolapse, acute coronary artery perforation, cardiac tamponade, artery dissection, serious ventricular arrhythmia, cardiac shock, no-reflow phenomenon, slow flow phenomenon, thrombus, hypersensitivity to contrast media which happened after PCI. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Normal systolic blood pressure (SBP) was defined as 90-120 mmHg. Normal heart rate was defined as 60-100 beats per minute. Hyperlipidemia was defined as total serum cholesterol (TC) ≥6.22 mmol/L, low-density lipoprotein cholesterol (LDL-C) ≥4.14 mmol/L, or triglyceride ≥2.26 mmol/L. Abnormal value of troponin T (TNT) was defined as ≥0.05 ng/mL. Creatinine clearance (Ccr) was calculated from serum creatinine (Scr) concentrations: Ccr (mL/min) = (140− age) × weight/[Scr (mg/dL) ×72] × (0.85 if patients is female).
Statistic analysis
To minimize the influence of other confounders on outcome, we used a propensity score analysis to match anemic patients with nonanemic patients. Anemic patients was matched in a 1:1 ratio with nonanemic patients using the nearest neighbor matching and based on gender, age, BMI, smoking status, drinking status, hypertension, arrhythmia, diabetes, hyperlipidemia, peripheral vascular diseases (PVD), history of cardiovascular and cerebrovascular diseases, family history of cardiovascular and cerebrovascular diseases, class of New York Heart Association (NYHA), number of diseased vessels, emergent PCI, vessel access for PCI, type of contrast agent, complete revascularization, SBP, heart rate, left ventricular ejection fraction (LVEF), TNT, Ccr.
We used t-test and χ2 test to compare variables between anemic and nonanemic patients including in pre-match and post-match models. Baseline variables demonstrating a significant association upon univariate analysis (P≤0.10 for inclusion) between 3-years ischemic events and anemia were entered into the multivariable model. Previously identified independent predictors of mortality were included in the model regardless of their strength on univariate correlation. Variables were selected by stepwise backward elimination and a P value <0.05 was considered significant. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for all included variables. Time to event data with estimated event rates determined according to the Kaplan-Meier method were compared with the log-rank test. We performed all statistical analyses using Stata 12.0 (Stata Corp, 2011).
Results
Baseline characteristics
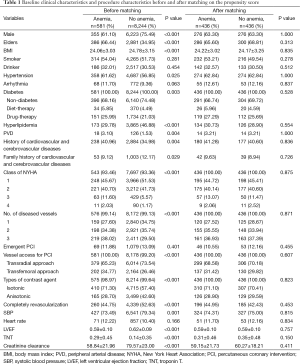
The baseline characteristics with and without anemia in the pre-match and post-match samples are presented in Table 1. Anemic patients were often female patients (38.90% vs. 14.51%) and more elder patients (66.44% vs. 34.95%), decreased BMI (24.06±3.03 vs. 24.78±3.15), higher prevalence of hypertension (61.62% vs. 56.85%), PVD (3.10% vs. 1.53%) or history of cardiovascular and cerebrovascular diseases (40.96% vs. 34.98%). Anemic patients were more often prone to multi-diseased vessels than nonanemic patients (38.02% vs. 29.50%). The LVEF (0.59±0.10 vs. 0.62±0.09) and Ccr (58.84±21.96 vs. 79.57±23.00) in anemic patients were less than nonanemic patients. In the post-match model, differences of all variables between anemic and nonanemic patients were reduced and had no statistical significance.
Full table
Clinical follow-up
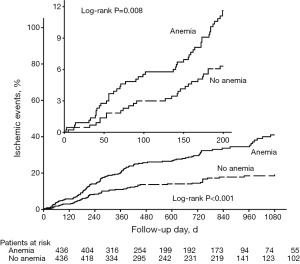
The incidence of 3-year ischemic events was 41.0% in anemic patients, 19.3% in nonanemic patients. The incidences of ischemic events in 6 months, 1 year, and 2 years were 9.3%, 20.3%, 30.0% for anemic patients, 6.1%, 11.3%, 14.9% for nonanemic patients (Figure 1). As is shown by Figure 1, the 3-year incidence of ischemic events was significantly higher in anemic patients. In fact, the incidence of ischemic events had become significantly higher in anemic patients since 200 days after PCI.
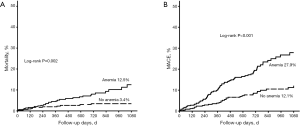
The incidences of 3-year mortality (12.5% vs. 3.4%, P=0.002) and 3-year MACE (27.9% vs. 12.1%, P<0.001) were also significantly higher in anemic patients than in nonanemic patients (Figure 2).
Multivariate analysis
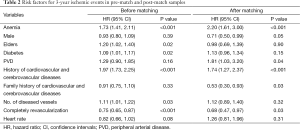
The analysis using the pre-match sample identified a positive relationship between anemia and ischemic events (HR: 1.73, 95% CI: 1.41-2.11, P<0.01). The pre-match analysis also showed significant association between ischemic events and the following covariates: elder age (HR: 1.21, 95% CI: 1.02-1.40, P=0.02), number of diseased vessels (HR: 1.11, 95% CI: 1.01-1.22, P=0.03), history of cardiovascular and cerebrovascular diseases (HR: 1.97, 95% CI: 1.73-2.25, P<0.01), diabetes (HR: 1.09, 95% CI: 1.01-1.17, P=0.02), completely revascularization (HR: 0.75, 95% CI: 0.65-0.87, P<0.01) (Table 2).
Full table
After controlling for all covariates using a propensity score matching (PSM) approach, anemia remained a significant predictor for ischemic events (HR: 2.20, 95% CI: 1.61-3.00, P<0.01). Similar to the results in the pre-match model, history of cardiovascular and cerebrovascular diseases (HR: 1.74, 95% CI: 1.27-2.37, P<0.01) and complete revascularization (HR: 0.68, 95% CI: 0.47-0.97, P=0.03) were also predictors for ischemic events. Male gender (HR: 0.93, 95% CI: 0.80-1.09, P=0.05), PVD (HR: 1.81, 95% CI: 1.03-3.20, P=0.04) and family history of cardiovascular and cerebrovascular diseases (HR: 0.53, 95% CI: 0.30-0.93, P=0.03) were shown to be significant predictors for ischemic events in the post-match model, but not in the pre-match model. Additionally, elder age, number of diseased vessels, diabetes were significant predictors in the pre-match model, but not in the post-match model (Table 2).
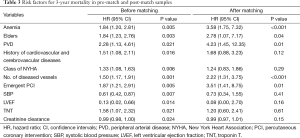
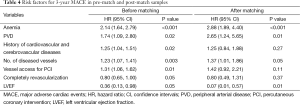
The relationships between covariates and 3-years mortality in the pre-match and post-match samples are shown in Table 3. Anemia was the predictor for 3-years mortality both in the pre-match model (HR: 1.84, 95% CI: 1.20-2.81, P<0.01) and in the post-match model (HR: 3.58, 95% CI: 1.75-7.32, P<0.01). Anemia was also the predictor for 3-year MACE in both pre-match model (HR: 2.14, 95% CI: 1.64-2.79, P<0.01) and post-match model (HR: 2.88, 95% CI: 1.89-4.40, P<0.01) (Table 4).
Full table
Full table
Discussion
The main and novel findings of this study include that (I) anemia is the independent risk factor for 3-year ischemic events and 3-year MACE in patients undergoing PCI; (II) the incidence of ischemic events had become higher in anemic patients since the 200 days after PCI. Our study is the first we know of using PSM to identify anemia as an independent risk factor for ischemic events, mortality and MACE in patients undergoing PCI.
Our findings agree with other studies relating anemia to ischemic events, mortality and CVD. Previous studies have shown anemia was associated with increased risk of a cardiovascular event in patients with CVD (11,28,29), including patients undergoing PCI (15,18-21,30,31).
CADILLAC trial demonstrated that anemia is strongly associated with increased disable stroke and mortality in patients undergoing PCI (20). What’s more, anemia was one of the seven predictors in the CADILLAC Risk Score to predict mortality (24) and Cho et al. (32) demonstrated combined use of Hb level and neutrophil-to-lymphocyte ratio (N/L) provides valuable timely information for early risk stratification in patients with ST-segment-elevation myocardial infarction (STEMI) undergoing primary PCI. Lee et al. (31) and Hosseini et al. (18) also showed an association between anemia and increased MACE and mortality after PCI. Among those studies, there were also studies that have identified the association of anemia and ischemic events in patients undergoing PCI. Kitai et al. (19), Nikolsky et al. (20), Bertrand et al. (33) showed that anemia is associated with increased risk of myocardial infarction, TVR. Although Katai et al. (19) and Nikolsky et al. (20) have shown anemia is associated with increased incidence of stroke, the definition of stroke in their studies also include hemorrhagic stroke. Although previous studies have shown that anemia have negative effects on the outcomes in patients undergoing PCI, few studies have done propensity matching to minimize the influence of other cofounders on outcomes, which can weaken the strength of evidence.
There are several potential reasons anemia may be a risk factor for ischemic events in patients who underwent PCI. First, the presence of anemia can decrease oxygen delivery to the myocardium and induce myocardial ischemia through mismatches in oxygen supply and demand, especially in patients with coronary artery stenosis (6-8). Even in case of correction of coronary artery stenosis with PCI, the existence of anemia may lead to a mismatch in oxygen supply and demand in the myocardium. This effect may induce an increased heart rate and blood volume that is mainly mediated through the activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system (34,35). If anemia extended for a long period, it may result in ventricular remodeling and cardiac dysfunction. Chronic anemia with Hb <10 g/dL is known to result in increased cardiac output that may lead to left ventricular hypertrophy (LVH) (36). The latter can sharpen the mismatch between supply and demand of oxygen, and thus increase the incidence of ischemic events. Second, the presence of anemia may in theory be a risk factor for myocardial ischemia. Although it is generally believed that lower levels of Hb are required to induce myocardial ischemia, this has not been rigorously studied. Third, reduced Hb may be associated with other risk factors for CVD that were not ascertained in this study, such as decreased nutritional status, additional measures of lower socio economic status, or increased inflammatory status. For example, an increased inflammatory status may indeed be the causal risk factor associated with ischemic events (37,38), with the presence of anemia simply being a marker of an underlying inflammatory process. Against the latter hypothesis is the fact that other putative markers of inflammation, such as fibrinogen, white blood cell count, factor VIII and von Willebrand factor, do not change the strength of the association between anemia and ischemic events (data is not shown).
There are also two unanswered questions that should be investigated in future studies. First, further studies need to assess laboratory factors or conditions that would help us ascertain the cause of the anemia, for example, subclinical liver disease or hemoglobinopathy, and indices of iron, folate or vitamin B12 deficiency. In theory, knowledge of these data may be instructive in suggesting potential mechanisms relating anemia to CVD. In fact, demonstration that iron deficiency anemia is a risk for CVD events would be counter to current thinking that suggests higher levels of iron may actually be a risk factor for atherosclerosis. Second, further studies need to explore that whether recovery of anemia can improve the outcome, especially the ischemic events, of anemic patients undergoing PCI.
Limitations
The limitations of our analyses are listed as follows: first, this post-hoc analysis was not pre-specified and should, thus, be considered hypothesis generating, and complementary to large, prospectively collected observational databases. We didn’t have data of many other cofounding factors which may also have impacts on the outcome in anemic patients, such as types of anemia, therapy for anemia or whether need blood transfusion. Therefore, we are unable to adjust for potential unmeasured confounding factors; second, previous studies showed that, recovery of anemia can significantly improve the outcome in general patients, we didn’t have relevant data to analyze the association of recovery of anemia with the outcome; third, our study is a single-center study, which may result in selection bias.
Conclusions
Anemia is an independent predictor for 3-year ischemic events, 3-year mortality and 3-year MACE in patients undergoing PCI. Further studies need to explore the impact of the pathogenesis and progress, prevention and therapy of anemia on the outcome of patients undergoing PCI.
Acknowledgements
Funding: This work was sponsored by non-profit grants from the Chinese Government National Key Research and Development Project for the 12th 5-year plan (2011BAI11B07).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mujica-Coopman MF, Brito A, López de Romaña D, et al. Prevalence of Anemia in Latin America and the Caribbean. Food Nutr Bull 2015;36 Suppl 2:S119-28. [PubMed]
- Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med 2004;116 Suppl 7A:3S-10S. [PubMed]
- Rachoin JS, Cerceo E, Milcarek B, et al. Prevalence and impact of anemia in hospitalized patients. South Med J 2013;106:202-6. [PubMed]
- Penninx BW, Pahor M, Woodman RC, et al. Anemia in old age is associated with increased mortality and hospitalization. J Gerontol A Biol Sci Med Sci 2006;61:474-9. [PubMed]
- Kikuchi M, Inagaki T, Shinagawa N. Five-year survival of older people with anemia: variation with hemoglobin concentration. J Am Geriatr Soc 2001;49:1226-8. [PubMed]
- Levy PS, Quigley RL, Gould SA. Acute dilutional anemia and critical left anterior descending coronary artery stenosis impairs end organ oxygen delivery. J Trauma 1996;41:416-23. [PubMed]
- Levy PS, Kim SJ, Eckel PK, et al. Limit to cardiac compensation during acute isovolemic hemodilution: influence of coronary stenosis. Am J Physiol 1993;265:H340-9. [PubMed]
- Maroko PR, Braunwald E. Effects of metabolic and pharmacologic interventions on myocardial infarct size following coronary occlusion. Circulation 1976;53:I162-8. [PubMed]
- Kulier A, Levin J, Moser R, et al. Impact of preoperative anemia on outcome in patients undergoing coronary artery bypass graft surgery. Circulation 2007;116:471-9. [PubMed]
- Muzzarelli S, Pfisterer M. TIME Investigators. Anemia as independent predictor of major events in elderly patients with chronic angina. Am Heart J 2006;152:991-6. [PubMed]
- Salisbury AC, Alexander KP, Reid KJ, et al. Incidence, correlates, and outcomes of acute, hospital-acquired anemia in patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2010;3:337-46. [PubMed]
- Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002;39:1780-6. [PubMed]
- Wu WC, Waring ME, Lessard D, et al. Six-month mortality and cardiac catheterization in non-ST-segment elevation myocardial infarction patients with anemia. Coron Artery Dis 2011;22:317-23. [PubMed]
- Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol 2002;40:27-33. [PubMed]
- Kim TH, Koh YS, Chang K, et al. Improved anemia is associated with favorable long-term clinical outcomes in patients undergoing PCI. Coron Artery Dis 2012;23:391-9. [PubMed]
- Leshem-Rubinow E, Steinvil A, Rogowski O, et al. Hemoglobin nonrecovery following acute myocardial infarction is a biomarker of poor outcome: a retrospective database study. Int J Cardiol 2013;169:349-53. [PubMed]
- Salisbury AC, Kosiborod M, Amin AP, et al. Recovery from hospital-acquired anemia after acute myocardial infarction and effect on outcomes. Am J Cardiol 2011;108:949-54. [PubMed]
- Hosseini SK, Ansari MJ, Lotfi Tokaldany M, et al. Association between preprocedural hemoglobin level and 1-year outcome of elective percutaneous coronary intervention. J Cardiovasc Med (Hagerstown) 2014;15:331-5. [PubMed]
- Kitai Y, Ozasa N, Morimoto T, et al. Prognostic implications of anemia with or without chronic kidney disease in patients undergoing elective percutaneous coronary intervention. Int J Cardiol 2013;168:5221-8. [PubMed]
- Nikolsky E, Aymong ED, Halkin A, et al. Impact of anemia in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: analysis from the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Trial. J Am Coll Cardiol 2004;44:547-53. [PubMed]
- Nikolsky E, Mehran R, Aymong ED, et al. Impact of anemia on outcomes of patients undergoing percutaneous coronary interventions. Am J Cardiol 2004;94:1023-7. [PubMed]
- McKechnie RS, Smith D, Montoye C, et al. Prognostic implication of anemia on in-hospital outcomes after percutaneous coronary intervention. Circulation 2004;110:271-7. [PubMed]
- Sabatine MS, Morrow DA, Giugliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 2005;111:2042-9. [PubMed]
- Halkin A, Singh M, Nikolsky E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol 2005;45:1397-405. [PubMed]
- Corona LP, Duarte YA, Lebrão ML. Prevalence of anemia and associated factors in older adults: evidence from the SABE Study. Rev Saude Publica 2014;48:723-431. [PubMed]
- Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004;104:2263-8. [PubMed]
- Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 2002;13:504-10. [PubMed]
- Matsue Y, Matsumura A, Abe M, et al. Prognostic implications of chronic kidney disease and anemia after percutaneous coronary intervention in acute myocardial infarction patients. Heart Vessels 2013;28:19-26. [PubMed]
- Peterson PN, Magid DJ, Lyons EE, et al. Association of longitudinal measures of hemoglobin and outcomes after hospitalization for heart failure. Am Heart J 2010;159:81-9. [PubMed]
- Kurek T, Lenarczyk R, Kowalczyk J, et al. Effect of anemia in high-risk groups of patients with acute myocardial infarction treated with percutaneous coronary intervention. Am J Cardiol 2010;105:611-8. [PubMed]
- Lee PC, Kini AS, Ahsan C, et al. Anemia is an independent predictor of mortality after percutaneous coronary intervention. J Am Coll Cardiol 2004;44:541-6. [PubMed]
- Cho KH, Jeong MH, Ahmed K, et al. Value of early risk stratification using hemoglobin level and neutrophil-to-lymphocyte ratio in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2011;107:849-56. [PubMed]
- Bertrand OF, Larose E, Rodés-Cabau J, et al. Incidence, range, and clinical effect of hemoglobin changes within 24 hours after transradial coronary stenting. Am J Cardiol 2010;106:155-61. [PubMed]
- Hébert PC, Van der Linden P, Biro G, et al. Physiologic aspects of anemia. Crit Care Clin 2004;20:187-212. [PubMed]
- Anand IS, Chandrashekhar Y, Ferrari R, et al. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br Heart J 1993;70:357-62. [PubMed]
- von Haehling S, Jankowska EA, van Veldhuisen DJ, et al. Iron deficiency and cardiovascular disease. Nat Rev Cardiol 2015;12:659-69. [PubMed]
- Steinvil A, Rogowski O, Banai S, et al. Anemia and inflammation have an additive value in risk stratification of patients undergoing coronary interventions. J Cardiovasc Med (Hagerstown) 2015;16:106-11. [PubMed]
- Siasos G, Tousoulis D, Athanasiou D, et al. Novel risk factors related to stable angina. Curr Pharm Des 2013;19:1550-61. [PubMed]