Pain control of thoracoscopic major pulmonary resection: is pre-emptive local bupivacaine injection able to replace the intravenous patient controlled analgesia?
Introduction
Video-assisted thoracic surgery (VATS) is known to have less pain compared to thoracotomy. However, pain is still not only a major concern for patients undergoing VATS but also considered as the main hindrance in fast postoperative recovery (1-3). Since the introduction of VATS lobectomy, the technique has become a routine procedure in major pulmonary resection (4,5). Despite the prevalence of VATS, there is still an ongoing debate concerning its appropriate pain control method (6-13). There are only a few studies comparing the efficacy of various pain control methods for VATS major pulmonary resection such as VATS lobectomy or segmentectomy (14,15).
Various methods have been proposed to relieve early postoperative pain after VATS. These modalities have included epidural PCA, intravenous patient controlled analgesia (IV PCA), pre-emptive local bupivacaine injection (PLBI), intraoperative intercostal nerve block with local anesthetics, continuous local analgesia to the thoracic surgical wound, and intermittent use of IV opioid or non-steroidal anti-inflammatory drug (NSAID).
Epidural PCA is commonly applied for thoracotomy patients. However, this most potent method is regarded as technically risky and time-consuming compared to other methods and it has not been clarified whether epidural PCA is necessary after VATS (8,15). IV PCA, a widely used and popular pain control modality for VATS, is a simple and convenient method. The effect of pain control is maintained continuously with stable therapeutic concentration. However, it is expensive and may accompany systemic side effects, such as nausea, vomiting, drowsiness, constipation, urinary retention, and in worst cases, respiratory depression. In addition, there is an issue of cost-effectiveness for IV PCA; considerable cases have experienced difficulty in maintaining IV PCA due to opioid induced side effects.
Alternatively, continuous-slow infusion of local anesthetics into the paravertebral or wound space with a catheter is being used for regional pain control modality. The superiority of paravertebral technique compared to epidural PCA in thoracotomy patients has been proven by Raveglia et al. via double blind, randomized controlled trial (16). However, there still exists a debate regarding its efficacy in VATS. Tunneling the paravertebral or extrapleural catheter at a proper position without tearing the parietal pleura is not easy and time-consuming under VATS. Technique related complications may occur, such as bleeding or delayed inflammation caused by additional injury to parietal pleura. If the catheter is placed in VATS wound space, local anesthetics can ooze from the wound due to small space. Furthermore, this is more expensive than IV PCA.
PLBI is performed immediately before skin incision under general anesthesia in the operation room (OR) and can be safely performed within 2 minutes. This long acting local anesthesia, bupivacaine, can theoretically cover acute pain within 24 hours and has relatively low-cost (0.5% bupivacaine 20 mL =1 vial =0.8 USD). Pre-emptive local anesthesia has already been used in simple minimally invasive thoracic surgery, such as wedge resection or sympathicotomy and proven to be effective in reducing postoperative pain (17,18). Theoretically, PLBI reduces the degree of sensitization produced in the nervous system by incision, retraction and trocar insertion. The nociceptive system perceives less pain compared to analgesia given after the occurrence of injury. Another advantage of PLBI is that it can be applied in specific areas, such as incision and chest tube insertion site, where it is considered most painful. The side effects, including low blood pressure due to anaphylaxis and wound infection are reported; however, the probability is very low.
The major issue concerning PLBI is whether there can be sufficient pain attenuation even in VATS major pulmonary resection without the use of conventional IV PCA. We hypothesized that PLBI may be an adequate pain control method even in VATS major pulmonary resection, as well as VATS minor procedures, if postoperative pain is expected to be significantly reduced 24 hours after surgery. The aim of this prospective randomized, open-label, non-inferiority trial was to evaluate whether PLBI may be able to replace IV PCA with fentanyl in VATS major pulmonary resection.
Materials and methods
Patient entry and randomization
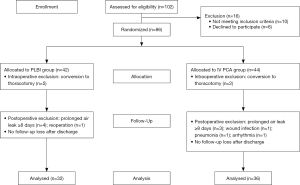
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (SNUBH study ID: B-1009-111-015, Clinical Trial ID: NCT01758809). From August 2010 to July 2011, patients who were scheduled to undergo lobectomy or anatomical segmentectomy by VATS at our institution were enrolled. Patients with significant preoperative chronic pain, such as headache or arthralgia, history of previous thoracic surgery, neoadjuvant chemo- or radio-therapy, serum creatinine level of more than 1.5, serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level of more than 120, and allergy to fentanyl or bupivacaine were excluded from this study. The exclusion criteria during an intraoperative or postoperative course were pulmonary wedge resection, conversion from VATS to thoracotomy, or prolonged hospital stay for more than 8 days due to air leak, pneumonia, acute lung injury, arrhythmia, wound infection, or reoperation.
At the time of entry, patients received an explanation about the study by a surgeon and a written informed consent was obtained. Patients who agreed to participate were randomly allocated to one of the two groups, IV PCA or PLBI group, while in the OR using a table of random numbers generated by a computer program.
A total of 86 patients were enrolled, with 18 patients (PLBI group, n=10; IV PCA group, n=8) being excluded as a result of intra- or post-operative course. Conversion to open thoracotomy was necessary in seven patients. There were nine patients with postoperative hospital stay of eight or more days due to prolonged air leak, pneumonia, or arrhythmia. One patient in the PLBI group who underwent reoperation and one with wound infection in the IV PCA group were also excluded from the study (Figure 1). There was no bupivacaine or fentanyl toxicity.
Anesthesia and operative procedure
All patients underwent total intravenous anesthesia of propofol and remifentanil within the target plasma concentration range of 3-5 µg/mL and 2-4 ng/mL, using the target controlled infusion pump (Orchestra® Base Primea, Fresenius Vial, France) without any induction drug. When the drug reached a level where the patient showed no sign of consciousness, we injected 0.6 mg/kg of rocuronium bromide. After confirming muscle relaxation, we performed intubation with a double-lumen endotracheal tube. Before the skin incision, the attending nurse allocated the patient through a table of random numbers to the IV PCA group or PLBI group, and the result was informed to the operator.
VATS lobectomy or segmentectomy was routinely performed via one lateral utility window (5-6 cm) and two access ports. We did not use any rib spreading or rib cutting. When performing VATS on the left side, an additional access port was occasionally needed. One 24 French chest tube was inserted through an access port after pulmonary resection. A foley catheter, which was maintained throughout the operation, was removed in the OR before transporting the patient to postanesthesia care unit (PACU).
Postoperative pain control
For the PLBI group, 3 cc of 0.5% bupivacaine in each interval of 1 to 2 cm along the incision line were infiltrated on the parietal pleura, muscle, and subcutaneous tissue. After bupivacaine wound infiltration, we rubbed the injection area for 1 to 2 minutes and waited for the drug to be fully absorbed before incision.
An IV PCA device, a continuous-infusion type with elastomeric pump, was connected to patients in the IV PCA group immediately after surgery. IV PCA bottle, which holds 100 mL of the solution, containing fentanyl 10 µg/mL, and was delivered (Accufuser Plus, Woo Young Medical, Chungbuk, Korea) with a basal infusion rate of 1 mL/h, bolus dose of 1 mL, and a lockout period of 15 minutes. If adequate postoperative pain relief could not be achieved, additional IV analgesics (morphine 5 mg or Ketolorac 30 mg) were administered in both groups. All patients were prescribed with tramadol 37.5 mg/acetaminophen 325 mg combination tablet (Ultracet™) every 6 hours as a regular medication if oral swallowing was possible. If the Ultracet™ induced side effects, including nausea/vomiting, dizziness, constipation, and urination difficulty, were suspected, switching medication to acetaminophen 650 mg was allowed.
Assessment of postoperative pain
All patients were transferred to the PACU after extubation to gain consciousness, stayed for approximately 1 hour, and moved to the general ward thereafter. The attending nurse checked the clinical status, including patient’s pain score, 4 times on the day of surgery (immediate after surgery in the PACU, arrival to the general ward, 4 hours after the transfer to the general ward and before going to sleep), and then every 8 hours thereafter. Pain intensity was recorded in accordance to the visual analogue scale (VAS) using Wong faces scale (range, 0-10; 0= no pain; 10= worst pain). VAS was measured serially at each stage from PACU to postoperative day (POD) 3, generally which was until chest tube removal. The pain score and the need of oral pain medication were followed-up in post-discharge 1 week, 1 month, and 2 months at the outpatient clinic.
If pain was checked as several times a day, the mean pain score was used to compare the two groups. Exceptionally, the pain score checked in the PACU was analyzed separately to show immediate postoperative pain. VAS rating at 0-3 was regarded as pain in effective control. In 4-6 rating, we allowed additional IV analgesics upon patient’s request. A VAS of 7 or more was regarded as intolerable pain and in need of additional analgesia.
Non-inferiority test and sample size
The study was designed as a non-inferiority test. Group sample sizes of 37 per group were calculated to detect non-inferiority using a one-sided, two-sample t-test with 80% power with a significance level (alpha) of 0.025. The data were drawn from previous study populations with standard deviations of 1.5 and 1.5, respectively (17). With an assumption of a 15% drop out rate throughout the study, 43 patients were planned for each group. The margin of non-inferiority was predefined as a mean VAS difference of less than 1.0 between the two groups. The primary end point was to compare the pain score between the two groups from POD 0 to POD 2 months. The secondary end point was to compare an additional number of IV analgesics during hospitalization, number of patients with analgesia induced side effects, and the need of oral analgesics after discharge.
Statistical analysis
Data were analyzed with SPSS 18.0 software program (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, USA). Continuous variables were presented as the mean ± SD and were compared using Student’s t-test; whereas categorical variables were evaluated using Fisher’s exact test. The VAS scores were compared using two-way repeated measures ANOVA. A P value of less than 0.05 was considered to indicate statistically significant difference.
Results
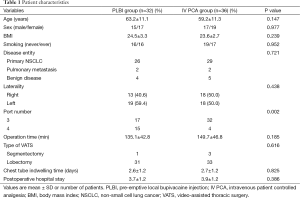
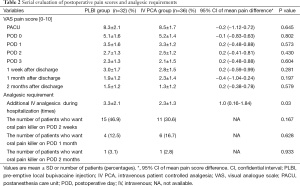
Data analysis was performed in 36 patients in the IV PCA group and 32 patients in the PLBI group. Patient characteristics are shown in Table 1. The mean age of PLBI patients was 63.2±11.1 and IV PCA patients was 59.2±11.3 years (P=0.147). There were no significant differences in sex, body-mass index, smoking status, disease entity, laterality, operation time, type of VATS, chest tube indwelling time, and hospital stay between the two groups. The percentage of patients with 4-port was higher in the PLBI group (P=0.002); this may be the case since there was a higher number of patients with left-sided VATS. The upper limit of the 95% confidential interval (CI) for the difference in the serial pain scores demonstrated no statistical differences between the PLBI and IV PCA groups [Immediate post-operation at PACU: 8.3±2.1 vs. 8.5±1.7, 95% CI of Δ pain score =−0.2 (−1.12-0.72), P=0.645; Day of surgery: 5.1±1.6 vs. 5.2±1.4, 95% CI of Δ pain score =−0.1 (−0.83-0.63), P=0.802; Day 1: 3.5±1.6 vs. 3.3±1.2, 95% CI of Δ pain score =0.2 (−0.48-0.88), P=0.573; Day 2: 2.7±1.3 vs. 2.5±1.2, 95% CI of Δ pain score =0.2 (−0.41-0.81), P=0.430; Day 3: 2.3±1.3 vs. 2.1±1.5, 95% CI of Δ pain score =0.2 (−0.48-0.88), P=0.604; 1 week after discharge: 3.0±1.7 vs. 2.8±1.5, 95% CI of Δ pain score =0.2 (−0.59-0.99), P=0.281; 1 month: 1.9±1.2 vs. 2.3±1.4, 95% CI of Δ pain score =−0.4 (−1.04-0.24), P=0.197 and 2 months: 1.5±1.2 vs. 1.3±1.2, 95% CI of Δ pain score =0.2 (−0.38-0.78), P=0.579] (Table 2).
Full table
Full table
In the PLBI group, the mean additional IV analgesics usage was one more compared to the IV PCA group during hospitalization (3.3±2.1 vs. 2.3±1.3; P=0.03). The requirements of oral analgesics in the outpatient clinic after discharge were investigated at three assessment time frames. Ultracet™, our standard oral analgesics for in-hospital and discharge, was prescribed in 26 patients (81.2%) of the PLBI group and 28 patients (77.8%) of the IV PCA group. Four patients (12.5%) of the PLBI group and seven patients (19.4%) of the IV PCA group discharged with acetaminophen 650 mg 3 times a day, due to tramadol related side effects. One patient (2.8%) of the IV PCA group and two (6.2%) of the PLBI group needed a more efficacious combination regimen of Ultracet™ plus NSAID. There was no statistical difference in oral analgesic regimen between the two groups (P=0.560). Patients who requested oral analgesics in the outpatient clinic on POD 2 weeks were 46.9% in the PLBI group and 30.6% in the IV PCA group. However, there was no statistical difference between the two groups (P=0.167). The number of patients requesting for oral analgesics on POD 1 month and 2 months also showed no statistical differences between the PLBI and IV PCA groups (POD 1 month: 12.5% vs. 16.7%, P=0.628; POD 2 months: 3.1% vs. 2.8%, P=0.933).
The side effects related with analgesics in each group are shown in Table 3. Patients who had no complaints of drug induced side effects in the PLBI group were 13 (40.6%) and 12 in the IV PCA group (33.3%; P=0.618). The occurrence of nausea/vomiting was higher in the IV PCA group compared to the PLBI group (12.5% vs. 38.9%; P=0.026), and 41.7% of IV PCA patients experienced opioid induced side effects that required removal of IV PCA within POD 1. The mean maintenance days of IV PCA were 1.9±1.0 days (range, 0-3 days).

Full table
Discussion
We set out to determine whether PLBI alone could be applied as a routine pain control method for VATS major pulmonary resection. A comparison between PLBI and IV PCA has proven that PLBI is simple, safe, effective and economical, and is applicable to VATS major pulmonary resection. The PLBI method was not associated with any wound infection or anaphylactic reaction.
There are a few limitations to our study. This study was not a double blind test and was not able to show a degree of pain in accordance to the areas of pain occurrence. Group sample sizes of 32 and 36, which were a little less than expected, achieved 77% power to detect non-inferiority. The number of patients enrolled in the study was relatively small to analyze the patient’s characteristics of those with side effects under the use of IV PCA. We also think the incidence of side effects may have differed if we modified the drug regimen or basal rate of device used for IV PCA.
The PLBI group had a higher number of patients who underwent 4-port VATS than the IV PCA group (46.9% vs. 11.1%; P=0.002). This can be interpreted in two following ways with regard to pain. First, the extra small port may help to decrease pain as this additional port reduces the torque of instruments in the intercostal space. Second, there is no significant difference between the two groups in terms of pain despite additional surgical trauma caused by an extra-incision. There is a limitation to the clear explanation. However, there is a lower risk of pain and paresthesia for using less ports, as verified by single port surgeries (19,20). Therefore, the use of more ports in the PLBI group may have been neglected in the interpretation of our results.
The concept of pre-emptive analgesia as a strategy to reduce the magnitude and duration of postoperative pain was introduced in 1983 by Woolf, who showed evidence for a central component of post-injury pain hypersensitivity in experimental studies (21). The spinal cord is being “wound up” by surgical trauma, despite general anesthesia. These nociceptive signals evoke a cascade of alterations in the somatosensory system, including an increase in the responsiveness of both peripheral and central neurons. These alterations increase the response to subsequent stimuli, amplifying pain. The preventive effect of pre-emptive analgesia for blocking the nociceptive transmission has the potential to reduce postoperative acute pain and occurrence of chronic pain as a result of central sensitization (22).
Previous studies (17,18) have reported the effectiveness of pain control in VATS minor surgeries using a pre-emptive local analgesia injection. Fiorelli et al. (17) conducted a prospective randomized double blind study by performing lidocaine injection on one side and normal saline on the other side before surgical incision when undergoing VATS sympathectomy. As a result, the side which received pre-emptive local analgesia showed significantly less pain in the first postoperative 24 hours compared to the control side, but not thereafter. We have also been controlling pain through PLBI without any use of IV PCA in simple procedures, including VATS wedge resection or sympathicotomy, and are satisfied with the results. However, in cases with longer operation time, more number of ports and longer utility window are required, like VATS lobectomy or segmentectomy, and most surgeons have a tendency to add pain control modality. Epidural PCA and IV PCA are esteemed as the two main pain control methods used in VATS. However, for years, there have been ongoing debates over the use of epidural PCA in patients who received VATS lobectomy because this method has been generally applied for the most painful procedures such as thoracotomy. A prospective randomized study performed by Kim et al. (14) evaluated the efficacy of epidural PCA and IV PCA among patients who received VATS lobectomy. They reported that there were no differences between the two groups in terms of pain scores, analgesic requirements, pulmonary function, satisfaction score, and the incidence of side effects. They went on to suggest IV PCA as an alternative pain control method for epidural PCA in patients who underwent VATS lobectomy. In addition, epidural PCA can be related to potential risks, such as dural perforation, bleeding, infection, hypotension, and urinary retention (23). Compared to IV PCA, it is time consuming and higher cost because a separate pain team participation is required (24). We have also selected IV PCA as the routine pain control method in VATS major pulmonary resection since we have determined that epidural PCA is unnecessary in VATS (15). However, IV PCA has frequently been accompanied with side effects such as nausea, vomiting, dizziness, and sleeping tendency, which led to discussions about the alternatives for IV PCA.
The most ideal pain control method for VATS major pulmonary resection must be simple, reduce acute pain developed within the first postoperative 24 hours, have almost no side effects, and be cost effective. We considered that PLBI can be an ideal pain control method for VATS, satisfying the aforementioned conditions. Our interest was in VAS at PACU immediately after the operation. A majority of patients required IV opioid at this point because both groups had a mean VAS greater than eight (PLBI: 8.3±2.1 vs. IV PCA: 8.5±1.7; P=0.645). VAS was higher than we expected at this time. The explanation is that the patient’s overall feeling of pain may not only be around the incision site, but also on the shoulder, urethra, throat, and other areas. However, after having the patients transferred to the general ward, pain maintained at a moderate range (VAS 4-6) and recovered fast at POD 2 to mild pain level (VAS 1-3). This result suggests that pain after postoperative 24 hours can be controlled only through oral pain medication for patients who undergo VATS lobectomy. An additional analgesic requirement showed an average of 1 more in the PLBI group than the IV PCA group (3.3±2.1 vs. 2.3±1.3, P=0.03). Nonetheless, considering the side effect incidence and uneconomical aspect of IV PCA, the result is deemed acceptable.
Pain control is important, but making efforts to minimize the side effects are also important. In our study, incidence of nausea/vomiting was 3 times higher in the IV PCA group (12.5% vs. 38.9%, P=0.026). In these cases, we had to stop the use of IV PCA and supplement with IV anti-emetics. For these reasons, 41.7% of patients needed a removal of IV PCA within postoperative 24 hours, which is a significantly high removal rate. Yoshioka et al. (8) explained that patients who received epidural PCA after VATS showed 6 times more incidence of nausea/vomiting than the non-epidural PCA group. For this reason, they advised to discontinue epidural PCA after POD 2. Kim et al. (14), in a study of VATS lobectomy patients, reported a 63% of nausea/vomiting incidence in the IV PCA group. In order to continue the use of fentanyl PCA in VATS, further studies must be done to reduce the incidence of nausea/vomiting.
When utilizing the PLBI method, enough amount of bupivacaine must be applied to infiltrate through the parietal pleura, muscle, and subcutaneous tissue, and an absorption time of 1 to 2 minutes must be allotted. In our study, we generally applied 20 cc of 0.5% bupivacaine on 3 or 4 VATS incision sites. We rubbed the injection site until the swelling completely subsided. This process is desired because when we rush an incision after PLBI, we have to wipe the unabsorbed bupivacaine with the gauze, and this may lead to a decrease of the analgesic effect.
Intercostal block with long acting anesthetics is commonly performed immediately before wound closure (25). However, such procedure, in addition to the PLBI method, was not used in this study because we thought pre-emptive injection was enough for pain control in VATS. Moreover, we could inject the drug sufficiently into the skin and muscle layers, as well as the extrapleural space. We regarded the injection of local anesthetics at the same level as repetitive work.
The problems of previous pain studies after VATS were as follows: the lack of serial pain score from right after surgery to discharge, no follow up data to check chronic pain at the point of POD 2 months, confounding data with VATS wedge resection, no standardization of oral pain medication, small number of enrolled patients. Our study measured VAS serially at each stage from PACU to POD 3, which was until chest tube removal. In addition, the pain score and the need of oral pain medication were followed-up in post-discharge 1 week, 1 month, and 2 months at the outpatient clinic. We also limited the study group into VATS lobectomy and segmentectomy patients, and strictly excluded patients who received wedge resection or any other minor resection surgeries. In order to minimize bias, we standardized the oral pain medication.
Conclusions
We were able to confirm significant alleviation of pain within 24 hours of operation in VATS major pulmonary resection. PLBI showed similar pain control effectiveness to IV PCA. However, IV PCA showed significantly higher side effects leading to a removal of the PCA device within POD 1. PLBI is a simple, safe, and low cost procedure. Therefore, PLBI is an alternative method, which is not inferior to IV PCA, even in VATS major pulmonary resection.
Acknowledgements
The authors thank Soyoung Chung and Sung Lee for editing this manuscript.
Funding: This work was supported by grant 02-2013-086 from the Seoul National University Bundang Hospital (SNUBH) Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Perttunen K, Nilsson E, Kalso E. I.v. diclofenac and ketorolac for pain after thoracoscopic surgery. Br J Anaesth 1999;82:221-7. [PubMed]
- Tschernko EM, Hofer S, Bieglmayer C, et al. Early postoperative stress: video-assisted wedge resection/lobectomy vs conventional axillary thoracotomy. Chest 1996;109:1636-42. [PubMed]
- Vogt A, Stieger DS, Theurillat C, et al. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesth 2005;95:816-21. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [PubMed]
- Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-22. [PubMed]
- Demmy TL, Nwogu C, Solan P, et al. Chest tube-delivered bupivacaine improves pain and decreases opioid use after thoracoscopy. Ann Thorac Surg 2009;87:1040-6; discussion 1046-7. [PubMed]
- Fernandez MI, Martin-Ucar AE, Lee HD, et al. Does a thoracic epidural confer any additional benefit following video-assisted thoracoscopic pleurectomy for primary spontaneous pneumothorax? Eur J Cardiothorac Surg 2005;27:671-4. [PubMed]
- Yoshioka M, Mori T, Kobayashi H, et al. The efficacy of epidural analgesia after video-assisted thoracoscopic surgery: a randomized control study. Ann Thorac Cardiovasc Surg 2006;12:313-8. [PubMed]
- Fibla JJ, Molins L, Mier JM, et al. The efficacy of paravertebral block using a catheter technique for postoperative analgesia in thoracoscopic surgery: a randomized trial. Eur J Cardiothorac Surg 2011;40:907-11. [PubMed]
- Hill SE, Keller RA, Stafford-Smith M, et al. Efficacy of single-dose, multilevel paravertebral nerve blockade for analgesia after thoracoscopic procedures. Anesthesiology 2006;104:1047-53. [PubMed]
- Forcella D, Pompeo E, Coniglione F, et al. A new technique for continuous intercostal-intrapleural analgesia in videothoracoscopic surgery. J Thorac Cardiovasc Surg 2009;137:e48-9. [PubMed]
- Taylor R, Massey S, Stuart-Smith K. Postoperative analgesia in video-assisted thoracoscopy: the role of intercostal blockade. J Cardiothorac Vasc Anesth 2004;18:317-21. [PubMed]
- Kaya FN, Turker G, Basagan-Mogol E, et al. Preoperative multiple-injection thoracic paravertebral blocks reduce postoperative pain and analgesic requirements after video-assisted thoracic surgery. J Cardiothorac Vasc Anesth 2006;20:639-43. [PubMed]
- Kim JA, Kim TH, Yang M, et al. Is intravenous patient controlled analgesia enough for pain control in patients who underwent thoracoscopy? J Korean Med Sci 2009;24:930-5. [PubMed]
- Kamiyoshihara M, Nagashima T, Ibe T, et al. Is epidural analgesia necessary after video-assisted thoracoscopic lobectomy? Asian Cardiovasc Thorac Ann 2010;18:464-8. [PubMed]
- Raveglia F, Rizzi A, Leporati A, et al. Analgesia in patients undergoing thoracotomy: epidural versus paravertebral technique. A randomized, double-blind, prospective study. J Thorac Cardiovasc Surg 2014;147:469-73. [PubMed]
- Fiorelli A, Vicidomini G, Laperuta P, et al. Pre-emptive local analgesia in video-assisted thoracic surgery sympathectomy. Eur J Cardiothorac Surg 2010;37:588-93. [PubMed]
- Sihoe AD, Manlulu AV, Lee TW, et al. Pre-emptive local anesthesia for needlescopic video-assisted thoracic surgery: a randomized controlled trial. Eur J Cardiothorac Surg 2007;31:103-8. [PubMed]
- Yang HC, Cho S, Jheon S. Single-incision thoracoscopic surgery for primary spontaneous pneumothorax using the SILS port compared with conventional three-port surgery. Surg Endosc 2013;27:139-45. [PubMed]
- Tamura M, Shimizu Y, Hashizume Y. Pain following thoracoscopic surgery: retrospective analysis between single-incision and three-port video-assisted thoracoscopic surgery. J Cardiothorac Surg 2013;8:153. [PubMed]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686-8. [PubMed]
- Dahl JB, Møiniche S. Pre-emptive analgesia. Br Med Bull 2004;71:13-27. [PubMed]
- Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology 1995;82:1474-506. [PubMed]
- Luketich JD, Land SR, Sullivan EA, et al. Thoracic epidural versus intercostal nerve catheter plus patient-controlled analgesia: a randomized study. Ann Thorac Surg 2005;79:1845-9; discussion 1849-50.
- Bolotin G, Lazarovici H, Uretzky G, et al. The efficacy of intraoperative internal intercostal nerve block during video-assisted thoracic surgery on postoperative pain. Ann Thorac Surg 2000;70:1872-5. [PubMed]


