A recurrent empyema with peripheral bronchopleural fistulas treated by retrograde bronchial sealing with Gore Tex plugs: a case report
Introduction
The development of peripheral bronchopleural fistulae (BPF) may be a serious complication of necrotizing pneumonia, and its treatment has been a challenge for thoracic surgeons. Although methods such as noninvasive bronchoscopy and surgery are available, they are not always successful and may not be appropriate for all patients. Here we describe a novel retrograde bronchial sealing technique using pieces of Gore-Tex graft material for multiple peripheral BPF in a 62-year-old man.
Case presentation
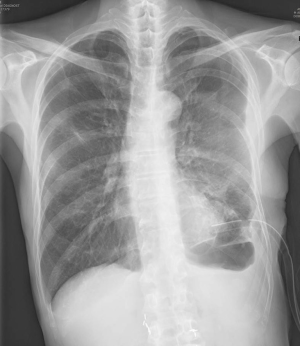
A very thin 62-year-old man was admitted after coughing up a large amount of purulent sputum and developing high fever. Chest radiography and computed tomography revealed necrotizing pneumonia in the left lower lobe. The patient had been diagnosed with Moyamoya disease and Buerger’s disease that included complicated claudication of the bilateral lower limbs 10 years ago. The causative factor for the necrotizing pneumonia was identified as Klebsiella pneumoniae, and the patient was successfully treated with antibiotics. However, he developed chronic peripheral BPF with recurrent empyema. Blood examination showed a hemoglobin concentration of 12.1 g/dL, white blood cell count of 10.2×103/mm3, and platelet count of 106×103/µL. Chest radiography (Figure 1) and CT showed two BPF and localized empyema with thickened pleura. Chest tube drainage and repeated chemical pleurodesis were ineffective.
We could not consider bronchoscopic intervention because of the high risk of ischemic insult that could lead to brain damage in association with Moyamoya disease and the presence of two relatively large BPF. The patient had briefly exhibited mental alterations during his first bronchoscopic procedure, and this was considered a sign of transient brain ischemic events that are generally caused by hyperventilation in patients with Moyamoya disease. Moreover, we could not resect the left lower lobe because of poor pulmonary function test results and severe pleural adhesion. Therefore, we performed decortication and primary suturing of the fistulae using fibrin glue and a pedicled intercostal muscle flap after precluding the use of a weak abdominal mesentery flap. However, the procedure failed to resolve the massive air leakage associated with BPF.
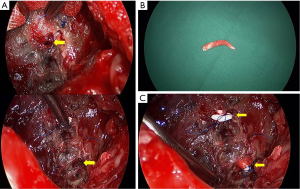
After 7 days, the recurrent pleural infection was controlled, and we decided to perform retrograde bronchial obstruction. We created a limited thoracotomy and performed aggressive debridement with irrigation. The two BPF were detected by a saline test. The diameter of these fistulae was 3.5-4 mm as measured by a gauged vascular guide. We rolled up small pieces of Gore-Tex (2 mm) and inserted them into the opened bronchus after BPF dilation using a vascular guide. The Gore-Tex plugs were fixed by deep sutures with 3-0 Prolene (Figure 2). Finally, the site was covered by the previously used intercostal muscle flap after confirmation of a negative saline test.
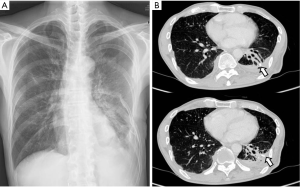
Immediately after surgery, the air leakage ceased. The patient’s postoperative course was uneventful, and he was discharged 7 days after the second surgery, at 81 days of hospitalization. Follow-up chest radiographs obtained 8 months later showed a small amount of pleural effusion with no evidence of recurrent BPF (Figure 3).
Discussion
We reported a case involving a 62-year-old man with multiple peripheral BPF as a complication of necrotizing pneumonia and described a novel retrograde bronchial sealing technique using pieces of Gore-Tex graft material for fistula repair. To the best of our knowledge, this is the first case treated by retrograde intrabronchial sealing of peripheral BPF using Gore-Tex plugs. BPF are a serious complication of necrotizing pneumonia, which is characterized by extensive parenchymal necrosis with fulminant pulmonary infection. The fistulae can be treated by surgery in select patients with progressive sepsis or simply controlled by antibiotic therapy when accompanied by empyema (1,2). In the present case, necrotizing pneumonia was controlled and stabilized with long-term antibiotic therapy; however multiple peripheral BPF with localized recurrent empyema developed soon after treatment. A retrospective radiology study demonstrated that lung abscess/necrotizing pneumonia, bronchiectasis, trauma, and malignancy are possible causes of peripheral BPF, and that surgical intervention should be used for patients with BPF that are visible on chest CT (3).
The management of empyema associated with BPF is a challenge for thoracic surgeons. The global treatment strategy for this complication is well known and comprises a three-pronged approach involving management of the fistula, pleural drainage, and antibiotic therapy. Treatment plans must consider the disease status, size and shape of the fistulae, and surgical feasibility. If a conservative approach is ruled out, the most important key to successful treatment is complete sealing of the fistulae. Several methods have been described for successful BPF sealing, including endobronchial interventions and surgery (1,4).
A number of case series and reports have described the use of one-way endobronchial valves for the treatment of BPF that develop after spontaneous pneumothorax, lung resection, and suppurative lung disease. In the largest reported series (40 patients), 93% patients experienced a decrease in air leakage, with 48% exhibiting complete resolution (5). For peripheral BPF, good outcomes have been reported with the use of bronchoscopic interventions using a sealing material such as fibrin glue, coils, or endobronchial valves. However, repeated procedures with or without simultaneous surgery for management of the empyema cavity may be required (6). Our patient was considered inappropriate for repetitive bronchoscopic procedures because of two relatively large peripheral BPF and Moyamoya disease (7).
Various surgical methods for BPF closure have been reported, including resection, resuturing, restapling, fibrin glue application, the use of a bovine pericardial patch that may be frequently accompanied by the use of a pedicled omental or muscle flap, and even thoracoplasty for closure of an infected pleural space (6,8). The first surgery for our patient involved the use of sutures, fibrin glue, and an intercostal muscle flap; however, this may be inadequate to resist expiratory bronchial pressure, particularly during coughing.
If the empyema accompanying BPF does not resolve despite all efforts, critical complications may arise in association with the long-term use of a chest tube or open drainage (9).
Because the pulmonary function of our patient was not sufficient to permit resection, and because suturing and fibrin glue application with a pedicled muscle flap failed to stop air leakage, we considered a new method to seal the multiple BPF. The Gore-Tex patch is a well-known artificial graft with lesser reactivity in the human body compared with that of other materials. However, it may aggravate pleural inflammation as a foreign body, similar to a bovine pericardium. We believed that intrabronchial plugs were less likely to aggravate the infection in our patient because the major portion of the plugs would be located within the bronchus, not the parenchyma. The plugs, fabricated by rolling Gore-Tex pieces, were required to remain stiff without any internal gaps, considering they would be tightly inserted along the course of small fistulae with thin elastic walls and seal them completely. Unfortunately, plugs fabricated from biological bovine pericardium do not exhibit these properties. The intercostal muscle flap used in the first surgery was used to cover the exposed Gore-Tex plugs in our patient. In addition, we sufficiently debrided the infected tissue in the pleural space, and consequently administered antibiotics to purify the drainage through a chest tube. Fortunately, empyema did not recur.
A similar bronchoscopy-guided technique using oxidized regenerated cellulose patch and fibrin glue was recently reported (10). This technique is considered suitable for large postoperative BPF without empyema that occur in the lobar or intermediate bronchus after major pulmonary resection surgeries. The location of the BPF enables the surgeon to apply the cellulose patch and inject fibrin glue into the bronchial wall for anchoring the patch. However, our patient exhibited two peripheral BPF accompanied by empyema that developed in subsegmental bronchi as complications of necrotizing pneumonia. As opposed to the cellulose patch used in the previous study, we employed retrograde insertion of peg-shaped plugs into the exposed fistulae to achieve complete surgical closure.
In conclusion, the findings from this case suggest that the retrograde sealing technique using Gore-Tex plugs for peripheral BPF with empyema is an effective alternative for the repair of selective peripheral BPF that cannot be treated by bronchoscopic interventions or other surgical treatments. However, it should be considered to avoid if the pleural inflammation was not controlled. Further accumulation of cases and studies are necessary to clearly demonstrate the effectiveness of this technique.
Acknowledgements
This work was supported by Chungbuk National University Grant in 2013.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gudbjartsson T, Helgadottir S, Ek L. One-way endobronchial valve for bronchopleural fistula after necrotizing pneumonia. Asian Cardiovasc Thorac Ann 2013;21:498-9. [PubMed]
- Tsai YF, Tsai YT, Ku YH. Surgical treatment of 26 patients with necrotizing pneumonia. Eur Surg Res 2011;47:13-8. [PubMed]
- Ricci ZJ, Haramati LB, Rosenbaum AT, et al. Role of computed tomography in guiding the management of peripheral bronchopleural fistula. J Thorac Imaging 2002;17:214-8. [PubMed]
- Jester I, Nijran A, Singh M, et al. Surgical management of bronchopleural fistula in pediatric empyema and necrotizing pneumonia: efficacy of the serratus anterior muscle digitation flap. J Pediatr Surg 2012;47:1358-62. [PubMed]
- Giddings O, Kuhn J, Akulian J. Endobronchial valve placement for the treatment of bronchopleural fistula: a review of the current literature. Curr Opin Pulm Med 2014;20:347-51. [PubMed]
- Nagata T, Nakamura Y, Kariatsumari K, et al. Experience of successful treatment for a case of intractable chronic empyema with a bronchopleural fistula. Kyobu Geka 2010;63:224-7. [PubMed]
- Spengos K, Tsivgoulis G, Toulas P, et al. Hyperventilation-enhanced chorea as a transient ischaemic phenomenon in a patient with moyamoya disease. Eur Neurol 2004;51:172-5. [PubMed]
- Tsai YM, Chen SL, Hsieh CM, et al. Treatment of empyema and bronchopleural fistula by bovine pericardium and latissimus flap. Ann Thorac Surg 2013;95:e39-40. [PubMed]
- Boudaya MS, Smadhi H, Zribi H, et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg 2013;146:575-9. [PubMed]
- Fiorelli A, Frongillo E, Santini M. Bronchopleural fistula closed with cellulose patch and fibrin glue. Asian Cardiovasc Thorac Ann 2015;23:880-3. [PubMed]