Rigid bronchoscopy and silicone stents in the management of central airway obstruction
Introduction
Rigid bronchoscopy has been an invaluable tool for over a century in the diagnosis and management of innumerable pulmonary diseases. Since its inception by Dr. Gustav Killian in the late 1800’s the use and popularity of this technique has waxed and waned. However, with advances in flexible bronchoscopy, ablative technologies and stenting over the past two decades rigid bronchoscopy has again become an integral tool in the management of malignant and non-malignant central airway disease by thoracic surgeons and interventional pulmonologists.
History of rigid bronchoscopy
Dr. Gustav Killian performed the first rigid bronchoscopy in the late 1800’s. This innovative procedure provided physicians with a new glimpse into human anatomy and sparked the growth of pulmonary medicine. Using a metal tube, a light, and topical cocaine anesthesia, Killian removed a pork bone from a farmer’s airway in 1897 (1). Prior to the invention of rigid bronchoscopy, over half of the patients who aspirated foreign bodies died, mostly of a post obstructive pneumonia. Rigid bronchoscopy with foreign body removal quickly evolved into the treatment of choice in these patients, with a clinical success rate above 98% (2). During the early 1900’s, Killian published extensively and lectured throughout the world. He further went on to adapt his bronchoscopes, laryngoscopes and endoscopes, and first described techniques such as using fluoroscopy and X-ray to define endobronchial anatomy. The design and functionality of the rigid bronchoscope was improved further in 1904 when Chevalier Jackson, who is regarded as the father of American bronchoesophagology first equipped his bronchoscope with a suction channel and a small light bulb at the distal tip to provide illumination.
The rigid bronchoscope quickly became an indispensable piece of equipment for otolaryngologists across the world and remained the only medical instrument to access the airways until 1963 when Shigeto Ikeda from the National Cancer Center Hospital in Tokyo, Japan introduced the flexible fiberoptic bronchoscope (3,4). For the next 30 years the use of rigid bronchoscopy declined as flexible bronchoscopy quickly gained worldwide acceptance and almost completely replaced the rigid bronchoscope as the diagnostic instrument of choice for pulmonary disease. In a survey performed in 1989, 8% of responders were performing rigid bronchoscopy (5). In a repeat survey in 1999 this number had declined to only 4% (6).
It was not until the lung cancer epidemic of the late 1900’s and the associated increase in central airway obstruction (CAO) that the utility of the rigid bronchoscope reemerged. In addition, recognition of certain advantages that rigid bronchoscopy has over flexible bronchoscopy such as airway control and ventilation during intervention as well as the ability to simultaneously use larger forceps, suction catheters and tumor excision techniques have led to the increase in rigid bronchoscopies being performed today.
Central airway obstruction (CAO)
The majority of rigid bronchoscopies done today are for the diagnosis and management of CAO. CAO is generally split into malignant and non-malignant disease. Malignant disease outnumbers non-malignant disease due to the rising incidence of primary lung cancer as well as countless types of malignancies that can metastasize to the lung (7). Non-malignant causes of CAO are often iatrogenic and secondary to endotracheal intubation or prior tracheostomy as well as inflammatory and connective tissue disorders (8). Although less prevalent, it is important to diagnose and treat these appropriately as patients with non-malignant CAO often have a longer life expectancy and can live with their disease for years to decades, as opposed to their malignant counterparts.
The incidence of CAO is difficult to estimate, but does appear to be on the rise in large proportion due to the increase in primary lung cancer. It is estimated that about 20-30% of patients with primary lung cancer will develop CAO, many of which may benefit from palliative measures to relive dyspnea caused by the obstruction (9). While less prevalent, non-malignant cases also appear to be on the rise. It is unclear whether this is due to increased availability of bronchoscopy leading to increased discovery versus the increase in ICU care, endotracheal intubation and tracheostomy (8).
The causes of malignant and non-malignant CAO are listed in Table 1. Primary tracheobronchial tumors are extremely rare consisting of adenoid cystic carcinomas [600-700 cases per year (10)], bronchial carcinoid tumors which are slightly more common with an incidence of 2 cases per 100,000 globally (11) and primary squamous cell and adenocarcinomas of the trachea. Much more common are metastasis of primary lung cancer, breast, renal cell, melanoma and thyroid cancers.

Full table
The anatomy of malignant CAO is generally classified into three groups. The first consisting of tumors that are purely intra-luminal and do not erode outside of the tracheal wall or cartilage. The second category of CAO is extrinsic compression from either parenchymal metastasis or mediastinal adenopathy. Finally, the most common category of CAO is a mixed extrinsic/intrinsic stenosis. These forms of stenosis generally originate outside of the airway and erode into the lumen. Differentiating between these three types of CAO is important as therapeutic options can differ for each category (Figure 1).
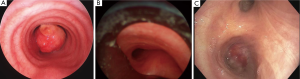
Non-malignant CAO is most frequently caused by iatrogenic injury post endotracheal intubation or tracheostomy placement. However, inflammatory and connective tissue disorders including Wegener’s granulomatosis, sarcoidosis, amyloidosis, relapsing polychondritis and tracheobronchopathia osteochondroplastica can also be the etiology of CAO. Another large category of non-malignant CAO is lung transplantation and stenosis at the anastomotic site. As mentioned previously the treatment intent in malignant CAO is almost always palliative in nature. This is certainly not the intent in many cases of non-malignant CAO where the intent should be curative. Rigid bronchoscopy with dilation and stenting should be used as a trial for improvement in symptomatology and as a bridge to curative surgical repair. Long-term stenting of benign stenotic airways may cause extension of the stenotic area due to granulation tissue, which may actually hinder future surgical repair.
As mentioned above the primary goal for rigid bronchoscopy is to relieve dyspnea and improve quality of life (QOL) caused by CAO in malignant as well as non-malignant disease. There have been a small number of papers published looking at the subjective improvement of dyspnea after bronchoscopy and relief of CAO, the largest being a multicenter registry study of 947 patients at 23 centers undergoing therapeutic bronchoscopy for CAO (12). Technical success was achieved on average in 93% of cases which was defined as opening of >50% of the airway. Dyspnea and QOL were measured using the Borg score and SF-6D in a portion of the study patients (187 and 183 respectively) with dyspnea subjectively improved in 48% of cases and QOL improved in 42%. Greater dyspnea at baseline as well as endobronchial obstruction was associated with greater improvement in dyspnea and QOL. American Society of Anesthesia (ASA) scores >3, renal failure, lobar obstruction, and tracheoesophageal fistulas were associated with less improvement.
The above trial like many of the other papers published on this topic, generally use a validated dyspnea or QOL score as the primary outcome. A recent prospective case series examined 53 patients undergoing therapeutic rigid bronchoscopy over a 3-year period and measured QOL and dyspnea scores as well as changes in pulmonary function testing (13). The population examined was predominantly malignant (45%) and post-transplant stenosis (43%) related CAO. Patients were found to have a subjective improvement of dyspnea and QOL measured by the University of San Diego Shortness of Breath Questionnaire and the SF-36 respectively, both validated tools to assess dyspnea and QOL in lung disease patients. Besides subjective improvements this study also showed a statistically significant improvement in FEV1 as well as FVC. There has yet to be any data showing improvement in survival after relief of malignant CAO.
Rigid bronchoscopy
Equipment
The rigid bronchoscope is a simple piece of equipment that has not significantly changed since its invention. Rigid bronchoscopes generally come in two forms; rigid tracheal scopes and rigid bronchoscopes. Both are hollow metal tubes with beveled distal edges available in several different diameters. The rigid bronchoscope is longer allowing access into the right and left bronchial trees as well as having distal fenestrations to allow ventilation of the contralateral bronchial tree. All scopes come with a built in or attachable port for jet or conventional ventilation. The light source can either be attached to the barrel, or more commonly directly to the camera. Scopes come in various sizes and length depending on the manufacturer, but all have an inner and outer diameter varying from 7-13 mm and 8-14 mm respectively.
Although there have not been significant advances in the rigid bronchoscope itself, countless types of instruments have been developed to use through the working channel. The first and most commonly used being the flexible bronchoscope. Once the airway has been secured with the rigid scope a flexible scope is often passed through the working channel and into segmental airways to lavage, suction secretions or blood as well as cannulate past smaller obstructions prior to coring, excising or stenting. A multitude of other instruments are now also available to pass down the working channel of rigid scopes including rigid and flexible suction catheters, different types and sizes of forceps, scissors, rigid and balloon dilators, multiple types of lasers, electrocautery, argon plasma coagulation and cryotherapy catheters, snares, loops, baskets, microdebriders, and stent deployment devices (14). Finally, a manual or automated jet ventilator is needed for oxygenation and ventilation during cases. If preferred or necessary the patient can also be ventilated by packing the mouth, placing silicone caps on the end of the rigid bronchoscope and using conventional positive pressure ventilation through a ventilator circuit adaptor placed on the proximal end of the rigid scope.
Rigid intubation and anesthetic considerations
Prior to rigid intubation the operator must discuss induction with the anesthesiologist, including medication choice and paralysis as well as a backup plan incase difficulty is encountered with rigid intubation. They must also ensure all equipment is ready and functional as well as properly position the patient to optimize first attempt success. Rigid bronchoscopy requires careful planning, cooperation and communication between the operator and the anesthesiologist to ensure patient safety.
Choice of induction agent is generally left to the anesthetist, depending on the patient’s medical history and clinical stability. Rigid intubation, similar to endotracheal intubation is extremely stimulating and generally deep sedation is required to blunt the gag reflex. Total intravenous anesthesia (TIVA) is almost exclusively used for rigid bronchoscopy, as a fully closed system required for inhaled anesthetics is rare. Generally a combination of a hypnotic administered simultaneously with a narcotic is used with the most common drugs being propofol (40-200 µg/kg/min) and remifentanil (0.05-0.5 µg/kg/min) (15). Similar to endotracheal intubation paralysis is not required during rigid intubation but can be helpful especially in patients with anterior or difficult airways. If paralysis is going to be administered the choice of agent should be discussed with the operator. When a short case is expected a shorter acting agent such as succinylcholine should be considered as a one-time dose or as an infusion. Otherwise, longer acting agents such as cisatracurium, vecuronium or rocuronium can be administered (15).
Once a plan for induction has been established and all equipment is ready the patient should be positioned with a shoulder roll or the head of the bed dropped to allow maximal extension of the neck without allowing the head to float. A tooth guard should be placed to protect the upper teeth, however if proper technique is used minimal to no pressure should be placed on the patient’s teeth. The operator should then scissor the patient’s mouth open in standard technique and carefully insert the rigid scope initially visualizing the tongue and hard palate. The operator’s thumb should be used to support the bottom of the rigid scope just outside of the oral cavity with the index and possibly the middle finger placed in the patient’s mouth resting on the hard palate. The scope is carefully inserted into the mouth with the bevel up or towards the tongue. The scope should then be carefully advanced further into the oral cavity until the uvula is visualized. Once the uvula is visualized the dominant hand holding the rigid scope and camera should be lowered using the thumb as a fulcrum and advanced further until the epiglottis is seen. The bevel of the rigid scope should then be used in a similar fashion as a Miller laryngoscope to lift the epiglottis anteriorly exposing the arytenoids, vocal cords and the glottis. If the patient’s glottis is anterior the thumb should be used to apply anterior and upward force on the rigid scope to expose the glottis. Care should be taken not to use the patient’s teeth as a fulcrum as this can cause significant damage. Once a good view of the glottis is achieved the scope is then rotated 90 degrees to allow the bevel to pass a traumatically through the vocal cords. The scope is then rotated another 90 degrees with the bevel rested on the posterior membrane of the trachea (16).
Once intubation is complete the jet ventilator is attached or the mouth is packed and ventilation is initiated and ensured by watching for chest rise prior to the initiation of any diagnostic or therapeutic interventions. The patient is monitored using continuous pulse oximetry, EKG tracing and blood pressure monitoring. Placement of an arterial line for blood pressure and blood gas analysis is generally not required unless the patient is unstable or a longer case is expected.
Ventilation techniques
Once rigid intubation is complete and the patient’s airway has been secured the operator must turn their attention to ensuring adequate oxygenation and ventilation. These are achieved with either jet ventilation or less commonly through conventional positive pressure ventilation. Sanders originally described open system manual jet ventilation in 1967, a technique still currently in use (17). The jet ventilator is connected to a 100% oxygen source and has a pressure-limiting device peaking at 50 PSI or less. The jet is connected through a catheter to the ventilation port of the rigid scope being used. Breaths are delivered to the patient at a rate of 12-18 per minute with the breath length and expiratory time being controlled by the anesthesiologist who is monitoring chest rise and vital signs to ensure adequate ventilation. The tidal volume of delivered air is dependent on the length of the breath given, the inspiratory pressure (PSI), the compliance of the patient’s respiratory system and the resistance of the patient’s airways (18). The advantage of jet ventilation is the ability to keep the working channel of the rigid bronchoscope completely open allowing easy passage of instruments. Disadvantages include the ability to only use 100% oxygen (unless an ambient air blender is available) as well as difficulty in oxygenating and ventilating sicker patients with severe parenchymal or obstructive lung disease.
A second type of jet ventilation commercially available is the automated jet ventilator. This is a computerized ventilator where the operator or anesthesiologist is able to set the applied pressure, respiratory rate, FiO2 and inspiratory time. The advantages of an automated system is the ability to free the anesthesiologist from holding the manual jet throughout the case as well as the ability to vary the FiO2 to enable the use of thermal ablative techniques without completely holding ventilation.
Closed system ventilation is achieved by attaching a ventilator circuit adaptor onto the distal end of the bronchoscope and using the rigid scope as an endotracheal tube. All of the proximal ports of the scope must be covered with silicone caps and the patient’s mouth must be packed with gauze to ensure minimal air leak. The advantage of this system is the ability to give positive pressure breaths through an automated ventilator circuit as well as positive end expiratory pressure (PEEP). This may allow improved ventilation and oxygenation in sicker patients with less respiratory reserve. A closed system also allows the use of inhaled anesthetics without significant exposure to those in the operating room. The disadvantages are the labor-intensive nature of packing the mouth as well as covering the proximal ports of the rigid scope with silicone caps, which can make the passage of multiple instruments difficult.
Tumor excision, coring and airway dilation techniques
After the patient has been successfully intubated with the rigid scope and ventilation has been initiated attention can be turned to relieving the CAO. Several different techniques can be utilized at this time and depends on the category of CAO encountered; extrinsic, intrinsic or mixed. For pure extrinsic compression without any mucosal or endobronchial involvement therapeutic intervention relies on CRE balloon and rigid dilation followed by silicone or covered metal stent placement to maintain a patent airway.
In the setting of intrinsic or mixed obstructions the initial goal is to establish patency of the airway. It is of critical importance that the operator maintains good visualization of the airway as well as appropriate spatial orientation to avoid perforation of the airway and invasion into the surrounding mediastinal structures. Once the operator has established good visualization, appropriate spatial orientation and a parallel axis to the central airway a decision can be made on how to remove the obstructing lesion. The first option is coring with the rigid scope, this allows one rotary forward motion to remove the lesion from the wall and simultaneously allows the scope to provide hemostasis at the site. Ensuring spatial and parallel axial orientation is of utmost importance during this method as one can easily core through an airway into the mediastinum (16). The next option includes mechanical excision with optical cup forceps, free forceps, loop cautery, microdebrider or a cryoprobe.
Microdebriders are powered instruments that consist of a hollow shaft with a rapidly rotating blade and suction. This device has been used for many years by the otolaryngology community for sinus and tracheal surgery (19). More recently, the procedure has been used as an alternative to or in conjunction with other modalities such as laser excision or electrocautery. Airway debulking with the microdebrider is accomplished by shaving and suctioning tissue under direct telescopic guidance through the rigid bronchoscope.
In a study by Lunn and colleagues, 16 subjects were treated with the microdebrider for the management of CAO. The majority of patients (87%) suffered from benign airway disease and remaining three patients (13%) had malignant airway obstruction. Using the microdebrider, the obstructing airway lesions were rapidly removed in all patients. There was only mild bleeding that occurred and was easily controlled by utilizing the rigid bronchoscope to tamponade the affected area or instillation of oxymetazoline hydrochloride. In this study, there were no procedure-related or long term complications of the microdebrider reported (20). A case report by Kennedy and colleagues reports the safe use of the microdebrider for more distal lesions due to the devices long length. The microdebrider was successfully used for relief of a distal left mainstem obstruction in a 59-year-old male with T3N2M1 non-small cell lung cancer followed by stent placement (21).
The oscillatory speed of the device and level of suctioning have varying adjustment levels and there have been reports in the literature of inadvertent resection of normal tissue with aggressive suctioning. There is one case report in the literature of pneumomediastinum and retroperitoneal air after the removal of tracheal papillomas with the microdebrider and jet ventilation. The author’s hypothesize those micro-perforations occurred within the tracheal wall in conjunction with jet ventilation and allowed air entry into the mediastinum and retroperitoneum. In this case, spontaneous resolution ensued without the need for surgical or medical intervention (22).
The microdebrider requires a rigid bronchoscope or laryngoscope and due to its rigid structure is not amenable to the flexible bronchoscopic technique. The advantages of the microdebrider for management of CAO include the ability to rapidly destroy and excise tissue with minimal bleeding, the ability to maintain a clear working field due to the automatic suctioning of the debrided tissue and no risk of airway fire or perforation. Although the technology appears promising and a useful modality in the management of CAO, further studies are required to assess the long-term outcomes of the microdebrider compared with more conventional therapy (14).
Cryotherapy is another safe and effective tool for debridement, hemostasis and removal of clot in rigid bronchoscopy. The device releases nitrous oxide or carbon dioxide stored under pressure into the tip of the cryoprobe which rapidly cools to −89 °C. The effectiveness of cryotherapy depends on the rapidity of the freezing and thawing process, the lowest temperature achieved the number of freeze-thaw cycles, and the water content of the tissue (23). Compared to other techniques for tumor destruction, the effects of cryotherapy are delayed, and frequently a repeat bronchoscopy to remove the necrotic tissue is required (24).
Cryotherapy has been used to successfully treat both benign and malignant CAO. It is effective in reducing or eliminating hemoptysis due to malignant disease in up to 93% of patients (25), and Maiwand and Homasson recommend cryotherapy as a first-line treatment in patients with post-transplant anastomotic strictures (26). As cartilage and fibrous tissue are relatively cryo-resistant, cryotherapy remains a very safe procedure. Bleeding is also uncommon because of the hemostatic effects of cryotherapy. Because of the lack of electrical current needed, cryotherapy is not associated with the risk of airway fires, electrical accidents, or radiation exposure. Cryotherapy can be used via both the rigid and flexible bronchoscopes. When using the flexible bronchoscope, care must be taken to have the probe protrude outside the distal tip of the scope, so as not to freeze the video chip.
After relief of the CAO due to an intrinsic or mixed lesion the operator must decide whether to leave the airway as is or if a stent is needed to maintain patency and stabilization. The risk of stent migration, granulation tissue formation and mucostasis must be weighed with the benefit of continued patency provided by the stent. This will be discussed extensively in the following section.
Silicone stenting
Introduction
Dr. William Montgomery is credited with initiating the widespread use of airway stents after his development of a silicone T-tube in 1965, but it wasn’t until 1990 that Dumon introduced that first completely endoluminal airway stent. The endobronchial stent remains the only tool available to alleviate extrinsic airway compression, but can be used in conjunction with other therapies to relieve CAO in those with intrinsic or mixed disease.
There are two main types of endobronchial stents available for use in the United States; silicone and metal. Silicone stents have been in use since the 1960’s and have a long track record of safety. Metal stents continue to evolve from the original uncovered metal stents to a number of newly designed covered metal stents made of nitinol. Unfortunately, the “ideal stent” has not yet been developed. This stent would be easy to insert and remove, yet not migrate; of sufficient strength to support the airway, yet be flexible enough to mimic normal airway physiology and promote secretion clearance; biologically inert to minimize the formation of granulation tissue; and available in a variety of sizes.
Types of silicone stents
Most of the commercially available silicone stents are based on the original Dumon stent, which is a silicone tube with external studs to decrease migration. The Dumon silicone stent comes in two main types’ straight and Y. Both types of these stents come in various lengths, diameters and shapes. The shape can be a uniform diameter throughout the length of the stent or have an hourglass shape with a narrow central portion allowing optimal positioning around a stenotic airway. Silicone stents can also have one end with a smaller diameter for optimal positioning. Y stents come with multiple various tracheal and bronchial limb diameters. These stents generally come in a uniform length, which can be adjusted at that time of insertion by shortening each of the three limbs to the desired length using a scalpel during the procedure. Other modifications can also be made at the time of insertion including the cutting of holes to allow ventilation of lobar bronchi covered by the stent during placement. Silicone stents can be made of transparent non-radio opaque material, or melted with barium sulfate, which are white in color, non-transparent but radio-opaque (27).
Another type of silicone stent commercially available currently is the Polyflex silicone stent (Boston Scientific, Natick, MA, USA). This stent is made of polyethylene threads embedded in a layer of silicone. These stents have a thin wall resulting in a better inner to outer diameter ratio compared to a Dumon stent. However, as they are not studded on the outside may have a higher rate of migration. They are embedded with tungsten making them radio-opaque and are deployed out of a semi-rigid tube inserted down a rigid bronchoscope. There are a small number of other companies commercially producing other types of silicone stents such as those manufactured by Hood (Hood Laboratories, Pembroke, MA, USA) with similar properties as the Dumon stent.
Dynamic Y tracheobronchial airway stents are also commercially available. These were initially described by Freitag et al. in 1994 (28) and are composed of anteriorly placed U shaped metal rings in the tracheal limb, with a silicone posterior membrane that can be dynamically compressed during cough to physiologically mimic the human trachea and allow for better mucus clearance. Finally, there are also numerous types of T-tubes and T-tube Y stent combinations manufactured currently for different types of tracheal and carinal pathology. This review will primarily focus on endobronchial silicone stents (27).
Indications
There are several indications for the use of silicone stents in the management of CAO. In general silicone stenting is indicated for maintaining central airway patency due to malignant and non-malignant disease causing greater than 50% stenosis of the trachea or bronchi. The first major indication includes stabilization of airways from malignant CAO. This can be either due to extrinsic compression, endobronchial tumor or a combination as described above. Stents may be the only resource available to maintain airway patency in extrinsic compression, as simple dilation is extremely transient if successful at all. In terms of endobronchial disease and mixed obstruction, stents are generally placed after excision and destruction of tumor has occurred to maintain patency and attempt to avoid recurrent obstruction by tumor re-growth or until systemic chemotherapy or radiation treatment has time to take effect. Post obstructive pneumonia caused by malignant airway obstruction is another appropriate indication, which may be necessary to maintain patency and provide adequate source control of the infected lobe or segment (29).
The second major indication is treatment of benign CAO caused by intubation trauma, tracheostomy, connective tissue disease, cartilaginous disorders or benign adenopathy. Silicone stents play an even larger role in benign disease, as they are preferred over metal stents due to complications with granulation of metal stents into the airway, difficulty with removal and fistula formation. Another benign indication for silicone stenting is stabilization of collapsing airways secondary to tracheobronchial malacia or cartilaginous disorders such as relapsing polychondritis. Finally, the last category of disease necessitating silicone stenting is to cover airway-esophageal or airway-mediastinal fistulas caused by malignant disease, iatrogenic complications of esophageal stenting, radiation therapy, or dehiscence of transplanted airways (29).
Deployment
Once a lesion has been dilated, excised or destroyed and the decision has been made to place a silicone stent several steps must be taken to ensure the appropriate stent is placed in a safe, accurate and timely manner. Deployment is still based on the original technique describe by Jean Francois Dumon in a 1990 paper published in Chest of 118 stents placed in 66 patients in Marseille, France (30).
The initial step prior to deployment is choosing the appropriate size and length of the stent to be placed. This is extremely important as proper sizing decreases the chance of migration (under-sizing) and the formation of granulation tissue, airway fistulization or difficulty in deployment (over-sizing). The length and diameter of a stent can be estimated using a chest CT prior to the procedure, but measurements during bronchoscopy are the only accurate way of sizing a stent. The diameter can usually be determined by choosing a size similar to the largest external diameter of the rigid bronchoscope used to maximally dilate the lesion in question. There are also commercially available stent sizers that can be placed down a rigid scope and used to measure the diameter of the stenotic airway. Once an appropriate size has been determined the length of the lesion should be measured using the rigid camera or flexible bronchoscope with the stent extending about 5-10 mm proximal and distal to the lesion. In sizing stents for fistulas, one should slightly oversize the stent as there is no endobronchial lesion to anchor the stent to, making migration more likely (27).
Once the appropriate stent has been selected it is loaded into a hollow metal stent deployment tube either manually or with a commercially available loading device. The rigid bronchoscope should then be placed slightly distal or within the stenosis. A prosthesis pusher is then placed through the hollow stent deployment tube and these are both placed down the rigid bronchoscope as ventilation is held. If the scope is positioned distal to the lesion, the rigid scope can be slightly withdrawn simultaneously as the stent is deployed to allow the stent to deploy within the stenosis. The deployment device is then removed and the camera alongside forceps should be placed back into the rigid scope. The stent may fully deploy, but generally requires being pulled back into optimal position, or slightly rotated to fully open. If manual rotation or adjustment does not fully open the stent the barrel of the rigid scope can be used to open the stent carefully without pushing it distally or a CRE balloon may be used to help expand the stent (30). If that is still unsuccessful, the stent is likely too large for the lesion and needs to be removed with a smaller diameter stent re-deployed.
Deployment of a Y silicone stent is slightly more difficult and can be accomplished through two general strategies. In both techniques the stent is loaded into the stent deployment device after lubrication with care taken to note the directionality in which the stent is folded in order to deploy the left and right limbs in the proper orientation. In the first technique the rigid bronchoscope is positioned above the carina based on the length of the tracheal limb. The stent is pushed out into the trachea in a similar fashion as described above and then using a camera, forceps and the rigid scope the stent is advanced and turned to position the left and right limbs appropriately in their respective mainstem bronchi flush with the carina.
In the second technique the stent is loaded in the same manner but deployed in the mainstem that will house the longer limb of the Y stent or alternatively the mainstem with the more stenotic airway. In this technique a rigid bronchoscope must be used and intubation of the right or left mainstem must be possible. Once the mainstem has been intubated the stent deployment device is inserted into the bronchoscope and the stent is pushed out of the device. As the stent is being deployed the rigid scope is slowly withdrawn back into the trachea until the stent is fully out of the deployer. The rigid telescopes as well as forceps are then used to pull the stent back gently from the mainstem it was deployed in, allowing the shorter limb to fall into place in the contralateral bronchus. Again rotation of the stent may be necessary to allow snug seating on the carina.
Deployment of a dynamic Y stent described in the previous section must be done in an altogether different manner than the two techniques described above. After inspection of the airway with a rigid or flexible bronchoscope and measurements made to determine the size of the dynamic Y stent to be placed the scope is removed and the patient ventilated with a bag valve mask or laryngeal mask airway until the stent is ready to be deployed. The dynamic Y airway stent is loaded onto a specific deployment device, which is a modification of rigid foreign body retrieval forceps, with longer jaws onto which the right and left mainstem limbs are inserted over. The operator then using direct laryngoscopy with either a Macintosh or Miller blade inserts the stent at a 90 degree angle carefully through the vocal cords on top of the deployment forceps using their right hand. Once the stent is fully inside the trachea, the stent is rotated 90 degrees clockwise and advanced until slight resistance is met. At this point the jaws of the forceps are opened and the stent is pushed further forward onto the carina (using a device connected to the deployment apparatus) until further resistance is met. At this point the jaws are closed and the deployment forceps withdrawn from the glottis. The patient is then rigidly intubated and the rigid camera and forceps are used to adjust the stent if necessary by further pushing it forward or rotating it to optimally seat it on the carina. The patient does need to tolerate a certain amount of apnea during deployment of a dynamic Y stent and the operator must be confident in their ability to obtain a good glottic view and rapidly re-intubate the patient with the rigid bronchoscope in the case of poor deployment and obstruction of the trachea by the stent. To aid in intubation or for training purposes, placement of dynamic Y stents with a video laryngoscope can be done to allow both the trainee and instructor to have a view of the glottis (31).
Complications
There are a wide range of complications related to rigid bronchoscopy and placement of silicone tracheal and bronchial stents ranging from as minor as mucostasis to death from hypoxic arrest during placement or migration of the stent. Complications from rigid bronchoscopy and stenting are in general very low, however when consenting a patient prior to a procedure a multitude of risks should generally be discussed including trauma to the oral cavity (lips, gums, teeth, tongue, pharynx), the vocal cords, the trachea and bronchi themselves, bleeding, infection, tracheal or bronchial rupture, hypoxia, respiratory failure requiring mechanical ventilation, tracheostomy, cardiac arrest and even death. If stenting is to be performed then the risks of stent placement are generally listed including mucus plugging, migration, formation of granulation tissue, bacterial overgrowth, halitosis and repeat procedures to inspect the stent, adjust if migrated and remove granulation tissue. Depending on the clinical stability of the patient different levels of emphasis can be placed on more serious complications such as respiratory failure and death. The overall risk and benefit of the procedure can then be discussed with the patient and family and an informed decision can be made to proceed, even in high-risk situations.
Most studies examining complications following bronchoscopic treatment of CAO have been retrospective in nature, however a recent multicenter registry trial published by Ost et al. examines 1,115 procedures performed on 947 patients at 15 centers from 2009 to 2013 (32). These included flexible (34%) and rigid (66%) therapeutic bronchoscopy under both general anesthesia (86%) and moderate sedation (14%) with multiple interventions reported including dilation (40%), ablative technologies: cryotherapy (8%), APC (35%), laser (23%) or electrocautery (21%) and stenting (36%). Only 44 patients (3.9%) were reported to have complications, which were defined as: bleeding requiring intervention, pneumothorax, hypoxemia, clinically significant airway injury, hypotension, arrhythmia, cardiac arrest, respiratory failure requiring mechanical ventilation and death. Of those reported complications 61% of those patients required a higher level of care. There were six deaths reported within 24 hours, four of which were secondary to a complication of the procedure and two unrelated. Two more deaths >24 hours after the procedure, but thought to be secondary to a complication of the procedure, were also reported. This gives a total mortality rate of 0.5% due to complications from therapeutic bronchoscopy.
Risk factors for complications included emergent procedures, ASA >3, re-do therapeutic bronchoscopy and the use of moderate sedation. Of note the use of neuromuscular blockade was associated with decreased rate of complications. This is likely due to the ability to oxygenate/ventilate better during the procedure as well as improved visualization due to lack of cough and patient movement. The study also examined risk factors for death at 30 days (14.8%) which were found to be associated with ASA >3, intrinsic or mixed obstructions or placement of a stent. The association with stenting and higher 30-day mortality is not likely due to the stent itself or stent complications, but rather to confounding factors including the fact that patients requiring stents likely have a higher level of disease burden are generally sicker and may not have other systemic treatment options remaining. Overall, the mortality/morbidity from therapeutic bronchoscopy is low when used in the appropriate setting.
Acknowledgements
We would like to acknowledge Drs. David Feller-Kopman and Hans Lee for their assistance with writing and review of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kollorath O. Entfernung eines Knochenstücks aus dem rechten Bronchus auf natürlichem Wege und unter Anwendung der directen Laryngoscopie. Münch Med Wochenschr 1897;38:1038-9.
- Becker HD, Marsh BR. History of the rigid bronchoscope. In: Bolliger CT, Mathur PN, editors. Interventional bronchoscopy, Progress in respiratory research. Karger: Basel, 2000;30:2-15.
- Alberti PW. The history of laryngology: a centennial celebration. Otolaryngol Head Neck Surg 1996;114:345-54. [PubMed]
- Ikeda S, Yanai N, Ishikawa S. Flexible bronchofiberscope. Keio J Med 1968;17:1-16. [PubMed]
- Prakash UB, Offord KP, Stubbs SE. Bronchoscopy in North America: the ACCP survey. Chest 1991;100:1668-75. [PubMed]
- Colt HG, Prakash UB, Offord KP. Bronchoscopy in North America: survey by the American Association for Bronchology, 1999. J Bronchol 2000;7:8-25.
- Lortet-Tieulent J, Soerjomataram I, Ferlay J, et al. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 2014;84:13-22. [PubMed]
- Grillo HC. The history of tracheal surgery. Chest Surg Clin N Am 2003;13:175-89. [PubMed]
- Ginsberg RJ, Vokes EE, Ruben A. Non-small cell lung cancer. In: DeVita VT, Hellman S, Rosenberg SA, ediors. Cancer: Principles and Practice of Oncology. 5th ed. Philidelphia: Lippincott-Raven, 1997:858-911.
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Uncommon primary tracheal tumors. Ann Thorac Surg 2006;82:268-72. [PubMed]
- Hemminki K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. Cancer 2001;92:2204-10. [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest 2015;147:1282-98. [PubMed]
- Mahmood K, Wahidi MM, Thomas S, et al. Therapeutic bronchoscopy improves spirometry, quality of life, and survival in central airway obstruction. Respiration 2015;89:404-13. [PubMed]
- Yarmus L, Ernst A, Feller-Kopman D. Emerging technologies for the thorax: indications, management and complications. Respirology 2010;15:208-19. [PubMed]
- José RJ, Shaefi S, Navani N. Anesthesia for bronchoscopy. Curr Opin Anaesthesiol 2014;27:453-7. [PubMed]
- Gordon J. Rigid Bronchoscopy. In: Ernst A, Herth FJ, editors. Principles and Practice of Interventional Pulmonology. New York: Springer, 2013:285-96.
- Sanders RD. Two ventilating attachments for bronchoscopes. Del Med J 1967;39:170-5.
- Pathak V, Welsby I, Mahmood K, et al. Ventilation and anesthetic approaches for rigid bronchoscopy. Ann Am Thorac Soc 2014;11:628-34. [PubMed]
- Kühnel T, Hosemann W, Rothammer R. Evaluation of powered instrumentation in out-patient revisional sinus surgery. Rhinology 2001;39:215-9. [PubMed]
- Lunn W, Garland R, Ashiku S, et al. Microdebrider bronchoscopy: a new tool for the interventional bronchoscopist. Ann Thorac Surg 2005;80:1485-8. [PubMed]
- Kennedy MP, Morice RC, Jimenez CA, et al. Treatment of bronchial airway obstruction using a rotating tip microdebrider: a case report. J Cardiothorac Surg 2007;2:16. [PubMed]
- Sims HS, Lertsburapa K. Pneumomediastinum and retroperitoneal air after removal of papillomas with the microdebrider and jet ventilation. J Natl Med Assoc 2007;99:1068-70. [PubMed]
- Asimakopoulos G, Beeson J, Evans J, et al. Cryosurgery for malignant endobronchial tumors: analysis of outcome. Chest 2005;127:2007-14. [PubMed]
- Mathur PN, Wolf KM, Busk MF, et al. Fiberoptic bronchoscopic cryotherapy in the management of tracheobronchial obstruction. Chest 1996;110:718-23. [PubMed]
- Marasso A, Gallo E, Massaglia GM, et al. Cryosurgery in bronchoscopic treatment of tracheobronchial stenosis. Indications, limits, personal experience. Chest 1993;103:472-4. [PubMed]
- Maiwand MO, Zehr KJ, Dyke CM, et al. The role of cryotherapy for airway complications after lung and heart-lung transplantation. Eur J Cardiothorac Surg 1997;12:549-54. [PubMed]
- Dutau H. Endobronchial SIlicone Stents for Airway Management. In: Ernst A, Herth FJ, editors. Principles and Practice of Interventional Pulmonology. New York: Springer, 2013:311-21.
- Freitag L, Eicker R, Linz B, et al. Theoretical and experimental basis for the development of a dynamic airway stent. Eur Respir J 1994;7:2038-45. [PubMed]
- Chin CS, Litle V, Yun J, et al. Airway stents. Ann Thorac Surg 2008;85:S792-6. [PubMed]
- Dumon JF. A dedicated tracheobronchial stent. Chest 1990;97:328-32. [PubMed]
- Yarmus L, Gilbert C, Akulian J, et al. Novel use of the GlideScope for rigid bronchoscopic placement of a Dynamic (Y) Stent. Ann Thorac Surg 2012;94:308-10. [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Complications Following Therapeutic Bronchoscopy for Malignant Central Airway Obstruction: Results of the AQuIRE Registry. Chest 2015;148:450-71. [PubMed]


