Prognosis and status of lymph node involvement in patients with adenocarcinoma in situ and minimally invasive adenocarcinoma—a systematic literature review and pooled-data analysis
Introduction
Lung cancer is the leading course of cancer-related mortality worldwide, and lung adenocarcinoma represents the most common histological subtype of lung cancer (1). In the past decade, numerous advances have taken place within various fields for lung adenocarcinoma, particularly in molecular biology with the discovery that EGFR and ALK biomarkers are responsive to targeted drugs. However, surgical resection is still the best option for patients with localized non-small cell lung cancer (NSCLC).
With the development of imaging procedures, such as high resolution computed tomography (HRCT), numerous small-sized lung cancers can be detected. Patients in which such small lesions are detected, generally have earlier disease stages at diagnosis, as a result, improved survival (2). Lobectomy with systematic lymph node dissection (SLND) is still the standard surgical treatment for complete resection (3); but, SLND remains controversial especially in the very-early stage NSCLC. Proponents argue that the removal of lymph nodes is important for survival as well as for staging. On the other hand, opponents consider that lymph node removal does not contribute to prognosis and may increase the morbidity and mortality after surgery. Therefore, the predictive factors of lymph node status are important for avoiding excessive surgery.
Recently, a new classification of adenocarcinoma was raised by the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) (4). The previous definition of BAC is no longer used and is instead called adenocarcinoma in situ (AIS) which is described as small (≤3 cm) solitary adenocarcinomas with pure lepidic growth lacking invasion. A related entity, sometimes previously referred to as minimally invasive BAC, is introduced in the new classification and called minimally invasive adenocarcinoma (MIA). MIA is described as small (≤3 cm) lepidic predominant tumors with ≤0.5 cm of invasion. A number of previous studies showed good survival prognosis for these two categories (5-7). Therefore, we hypothesized limited surgery that avoids excessive lymph node dissection might be appropriate for patients with AIS and MIA.
We performed a systematic literature review and pooled data analysis focusing on the lymph node status and survival prognosis after curative surgery for patients with AIS and MIA and describe our results here.
Methods
Literature search
Medline by PubMed, Springer and Ovid databases were used to search for relevant studies from the date of inception to April 2015. The language was limited to English. We used “adenocarcinoma in situ”, “minimally invasive adenocarcinoma”, “bronchioloalveolar carcinoma” and “lung adenocarcinoma” in different combinations in all fields. Reference lists of relevant publications were subsequently searched as a supplement.
Study selection
All studies containing patients with AIS or MIA were selected. Because the new classification of lung adenocarcinoma by IASLC/ATS/ERS was only implemented in 2011, we also included the former pure-BAC with tumor ≤3 cm and Noguchi classification (8) of localized BAC (less than 2 cm in diameter) without active fibroblastic proliferation (types A and B) for analysis.
Included studies met the following criteria: (I) studies included patients with AIS or MIA or pure-BAC ≤3 cm or Noguchi type A and type B adenocarcinoma; (II) all patients should underwent curative surgical treatment; (III) included patients did not receive adjuvant chemotherapy and radiotherapy before or after surgery; (IV) the surgical procedure and the situation of lymph node metastasis were clearly stated; (V) studies were limited to human subjects. Studies that included only pathologic-N0 patients were excluded. Abstracts, case reports, conference presentations, editorials and expert opinions were excluded.
Outcome measures
Primary outcome was the number of included patients with lymph node metastasis; 5-year disease free survival (5-year DFS) rates were also assessed.
Data extraction and quality assessment
The full-text articles were reviewed and data were extracted by two independent authors. Any discrepancies were resolved by discussion and came to consensus with a third author. Extracted data included: publication details, study period, median follow-up time, number of included patients, surgical procedure, 5-year DFS rates. The quality of observational studies was modified by criteria suggested by the Newcastle-Ottawa quality assessment tool (9).
Results
Literature search
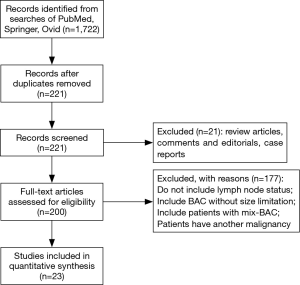
A total of 1,722 articles were found after the initial literature search. After implementing inclusion and exclusion criteria, and removal of duplicate publications, review articles, case reports, conference presentations, editorials and chapters of books, a total of twenty-three studies were eligible for further analysis (5,7,8,10-29) (Figure 1). Between the 23 articles, there were 6,137 lung adenocarcinomas, of which AIS/MIA accounted for 821. All included patients received curative surgical resection. None of the included patients received adjuvant chemotherapy or radiotherapy before or after surgery. Details are listed in Table 1.
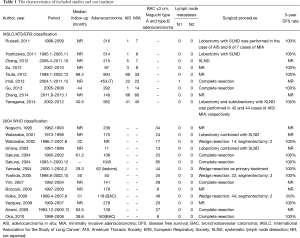
Full table
Detection of N disease, 5-year DFS
Patients with AIS/MIA underwent lobectomy, sublobectomy or wedge resection in combination with either lymph node sampling or SLND in these studies. After a review of the lymph node status, only one N1 metastasis was found in MIA, and no cases of N2 disease were found. Studies that reported 5-year DFS have almost 100% DFS rate (Table 1). We therefore propose that patients with AIS/MIA have extremely low rates of metastasis and recurrence.
Discussion
To the best of our knowledge, the present study is the first and largest analysis in regard to lymph node status in patients with AIS and MIA. In the new classification of lung cancer (4), the definition of BAC is no longer used and instead divided into five major categories: (I) AIS; (II) MIA; (III) lepidic predominant adenocarcinoma (nonmucinous); (IV) other invasive adenocarcinomas with lepidic component; (V) invasive mucinous adenocarcinoma. In our study we analyzed two kinds of low grade adenocarcinoma: AIS, which is a localized small (≤3 cm) adenocarcinoma with growth restricted to neoplastic cells along preexisting alveolar structures (lepidic growth), lacking invasion; and MIA, which is a small, solitary adenocarcinoma (≤3 cm), with a predominantly lepidic pattern and ≤5 mm invasion in greatest dimension in any one focus. Patients defined under the classification of lung adenocarcinoma by Noguchi and associates (8) (less than 2 cm in diameter), localized BAC without active fibroblastic proliferation classified as types A and B, were also analyzed in the present study.
The combination of all 23 studies held 6,137 lung adenocarcinoma cases, of which the AIS/MIA accounted for 821. Because some studies only included patients with adenocarcinoma 2 cm or less (8,14,17,19,20), the percentage of AIS/MIA reached 14% in our research. All patients with AIS/MIA received curative surgery combined with either lymph node sampling or SLND. From the results we found that only one patient with MIA had N1 lymph node metastasis, no cases of N2 disease were found. Although overall survival is the most common survival analysis method utilized in lung cancer studies, DFS more accurately reflects the biological behavior of the tumor in low grade tumor populations such as our series of AIS/MIA, where there is a high percentage of overall survival. Studies that reported 5-year DFS have almost 100% DFS rate; the recurrence of AIS/MIA was extremely low.
With the application of HRCT screening, an increasing number of early-stage lung cancers have been detected, most of which are AIS/MIA. AIS is also known to correspond to pure ground-glass nodule (GGN), MIA was more commonly referred to as mix-GGN (14,30). Forecasting lymph node metastasis is very important in determining the treatment strategy. The definition of complete resection, proposed by the IASLC, mandates that: a negative microscopic margin is obtained and SLND should be performed (3). However, previous studies demonstrated that survival of lymph node sampling was acceptable and was no worse than that after SLND (31,32). The American College of Surgery Oncology Group Z0030 trial (33) showed no difference in both survival and DFS between SLND and sampling group. Meanwhile, there was a significantly longer mean duration of surgery and a higher drain secretion in the SLND group compared with that in the sampling group (34,35). For patients with no risk or with ultralow risk of recurrence, excessive surgery or unnecessary therapy may be avoided. Based on these data and analysis, we believe that limited surgical resection without systemic lymph node sampling or dissection is adequate surgical treatment for the treatment of pathologic AIS/MIA NSCLC.
However, the results regarding AIS/MIA as very favorable prognostic categories have largely been based on nonmucinous tumors, as mucinous and mixed mucinous/nonmucinous AIS/MIA are extremely rare. Kadota et al. (36) and Noguchi et al. (8) reported mucinous AIS and MIA that had 100% 5-year DFS. Oka et al. (29) found that patients with mucinous AIS/MIA showed excellent prognosis after surgical resection, none of the patients had lymph node metastases or lymphovascular invasion. Hence, recent results showed no difference of survival benefits between mucinous and nonmucinous AIS/MIA.
Recently, in a report presented by Yeh et al. (37), frozen section was found to have a specificity of 80% in identifying lepidic predominant adenocarcinomas. In cases of AIS, all pathologists made accurate interpretations using frozen sections. In cases of MIA, 6.7% of frozen section diagnoses were AIS, 41.3% were MIA, and 52% were invasive adenocarcinoma. Also the inflation method, as described by Myung et al. (38) and Xu et al. (39) has been shown to be able to expand the alveolar spaces in frozen section slides. It did show a promise of frozen section in classifying lung adenocarcinoma subtypes. We believe that multiple frozen sections to classify lung adenocarcinoma subtypes intraoperatively would increase the concordance rate.
A number of limitations should be noted. First, because the new classification was brought up in 2011, some patients might have been missed as we only included pure-BAC with tumor ≤3 cm and Noguchi type A and B; second, the lymph node disposition was not unanimous among the included studies; third, all results came from postoperative pathological analysis; forth, not all included studies reported 5-year DFS rate and median follow-up; fifth, the number of harvested lymph node is not reported in all included papers. They only tell whether SLND is used. We listed this information together with surgical procedure in the table.
Conclusions
These results indicated that patients with AIS/MIA have good survival prognosis after surgical treatment. Because of the rare recurrence and lymph node metastasis rates, patients with these very-early stage NSCLCs could be nominated for limited surgical resection that SLND or sampling might be avoided.
Acknowledgements
We would like to thank Ms. Lindsey Hamblin to help edit the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:593-602. [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg 2014;98:453-8. [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [PubMed]
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [PubMed]
- Zhang Y, Sun Y, Shen L, et al. Predictive factors of lymph node status in small peripheral non-small cell lung cancers: tumor histology is more reliable. Ann Surg Oncol 2013;20:1949-54. [PubMed]
- Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. [PubMed]
- Imai K, Minamiya Y, Goto A, et al. Bronchioloalveolar invasion in non-small cell lung cancer is associated with expression of transforming growth factor-β1. World J Surg Oncol 2013;11:113. [PubMed]
- Zhang Y, Qiang JW, Ye JD, et al. High resolution CT in differentiating minimally invasive component in early lung adenocarcinoma. Lung Cancer 2014;84:236-41. [PubMed]
- Gu J, Lu C, Guo J, et al. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol 2013;107:474-80. [PubMed]
- Xu L, Tavora F, Battafarano R, et al. Adenocarcinomas with prominent lepidic spread: retrospective review applying new classification of the American Thoracic Society. Am J Surg Pathol 2012;36:273-82. [PubMed]
- Watanabe S, Oda M, Go T, et al. Should mediastinal nodal dissection be routinely undertaken in patients with peripheral small-sized (2 cm or less) lung cancer? Retrospective analysis of 225 patients. Eur J Cardiothorac Surg 2001;20:1007-11. [PubMed]
- Watanabe S, Watanabe T, Arai K, et al. Results of wedge resection for focal bronchioloalveolar carcinoma showing pure ground-glass attenuation on computed tomography. Ann Thorac Surg 2002;73:1071-5. [PubMed]
- Ishiwa N, Ogawa N, Shoji A, et al. Correlation between lymph node micrometastasis and histologic classification of small lung adenocarcinomas, in considering the indication of limited surgery. Lung Cancer 2003;39:159-64. [PubMed]
- Sakurai H, Maeshima A, Watanabe S, et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol 2004;28:198-206. [PubMed]
- Sakurai H, Dobashi Y, Mizutani E, et al. Bronchioloalveolar carcinoma of the lung 3 centimeters or less in diameter: a prognostic assessment. Ann Thorac Surg 2004;78:1728-33. [PubMed]
- Yamada S, Kohno T. Video-assisted thoracic surgery for pure ground-glass opacities 2 cm or less in diameter. Ann Thorac Surg 2004;77:1911-5. [PubMed]
- Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005;129:991-6. [PubMed]
- Yim J, Zhu LC, Chiriboga L, et al. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol 2007;20:233-41. [PubMed]
- Borczuk AC, Qian F, Kazeros A, et al. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. Am J Surg Pathol 2009;33:462-9. [PubMed]
- Koike T, Togashi K, Shirato T, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination. Ann Thorac Surg 2009;88:1106-11. [PubMed]
- Vazquez M, Carter D, Brambilla E, et al. Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer 2009;64:148-54. [PubMed]
- Anami Y, Iijima T, Suzuki K, et al. Bronchioloalveolar carcinoma (lepidic growth) component is a more useful prognostic factor than lymph node metastasis. J Thorac Oncol 2009;4:951-8. [PubMed]
- Oka S, Hanagiri T, Uramoto H, et al. Surgical resection for patients with mucinous bronchioloalveolar carcinoma. Asian J Surg 2010;33:89-93. [PubMed]
- Lederlin M, Revel MP, Khalil A, et al. Management strategy of pulmonary nodule in 2013. Diagn Interv Imaging 2013;94:1081-94. [PubMed]
- Jiang W, Chen X, Xi J, et al. Selective mediastinal lymphadenectomy without intraoperative frozen section examinations for clinical stage I non-small-cell lung cancer: retrospective study of 403 cases. World J Surg 2013;37:392-7. [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg 2006;81:1028-32. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [PubMed]
- Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290-4; discussion 294-5. [PubMed]
- Ma K, Chang D, He B, et al. Radical systematic mediastinal lymphadenectomy versus mediastinal lymph node sampling in patients with clinical stage IA and pathological stage T1 non-small cell lung cancer. J Cancer Res Clin Oncol 2008;134:1289-95. [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [PubMed]
- Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ≤ 3 cm: accuracy and interobserver agreement. Histopathology 2015;66:922-38. [PubMed]
- Myung JK, Choe G, Chung DH, et al. A simple inflation method for frozen section diagnosis of minute precancerous lesions of the lung. Lung Cancer 2008;59:198-202. [PubMed]
- Xu X, Chung JH, Jheon S, et al. The accuracy of frozen section diagnosis of pulmonary nodules: evaluation of inflation method during intraoperative pathology consultation with cryosection. J Thorac Oncol 2010;5:39-44. [PubMed]