Significance of serum cardiac troponin I levels in pulmonary embolism
Abstract
Background: Some biomarkers can be helpful in the diagnosis of pulmonary embolism (PE) and determining of severity
and prognosis of the disease. In this study, we aimed to analyze the elevated cardiac troponin I (cTnI) levels and its
association with electrocardiography (ECG) and transthoracic echocardiography (TTE) findings in patients with PE.
Methods: Totally 106 patients with suspected PE were included in the study. PE was confirmed in 63 of them, whereas
it was excluded in the remaining 43 patients. Levels of cTnI were measured in all patients before the prescription of the
anticoagulation therapy.
Results: High cTnI levels were found in 50.8% of patients with PE, and in 11.6% of patients without PE (P<0.001).
Sensitivity and specificity of the test for the diagnosis of PE were 50.7%, 88.3% respectively. ECG findings were similar in
PE patients having either elevated or normal cTnI levels. Approximately 75% of the PE patients with high cTnI had normal
ECG findings; the most common pathological changes seen in ECG were S1Q3T3 pattern (~31%). TTE findings were not
found to be distinguishing in the patients with suspected PE and high cTnI levels. Pulmonary hypertension (PHT) was the
most common echocardiographic finding (~74%) in patients with PE and elevated cTnI levels. However, there was not a
statistically significant difference between TTE findings in PE patients with increased and normal cTnI levels.
Conclusions: In patients presenting with clinical, electrocardiographic and echocardiographic features suggesting
pulmonary embolism, increased serum cTnI levels endorse the diagnosis of severe PE.
Key words: Cardiac troponin I; echocardiography; electrocardiography; pulmonary embolism
Introduction
Pulmonary embolism (PE) is the occlusion of the pulmonary artery or its branches with various substances such as thrombus, fat, air, bone marrow, amniotic fluid and septic material. It is commonly originated from the thrombi in the deep leg veins (1).
Cardiac troponins are the most sensitive and the most specific biochemical markers showing myocardial injury. Elevated troponin can also be seen in acute pericarditis, myocarditis, acute PE, severe cardiac failure, sepsis and acute renal failure as well as in myocardial infarction (2,3).
In acute PE, the mechanic load of the right ventricle is increased because of the increased pulmonary vascular resistance following pulmonary artery obstruction. That can lead to an acute right ventricle dilatation. The dilatation and hypokinesia of the right ventricle may cause severe myocardial ischemia and increase the troponin levels (4).
In this study including patients with PE, we aimed to investigate the relationship between serum cTnI levels and the severity of the disease by use of ECG and TTE.
Materials and methods
This study was planned as a prospective, case control, clinical trial. A local ethics committee approval was received for the study. One hundred and six patients evaluated for suspected PE in the clinic of pulmonary diseases of a university hospital in the period between February 2006 and February 2008 were included in the study. Patients were excluded if the clinical symptoms were judged to be caused by sepsis, hypovolemia, or chronic renal failure. Patients admitted with angina pectoris were also excluded, as were all patients with electrocardiographic findings suggesting ischemic heart attack.
Blood samples and laboratory methods
Blood samples were obtained on presentation in our hospital. Measurements of serum markers were performed by laboratory personnel blinded to the clinical data. Cardiac troponin-I (cTnI) was measured with the Access AccuTnI® assay (Beckman Coulter Inc), which is a chemiluminescent immunoenzyme method with two antibodies (sandwich type). According to the manufacturer, a cTnI level higher than 0.01 ng/dL was considered as elevated.
Investigations
All consecutive patients in whom the diagnosis of pulmonary embolism was made underwent echocardiographic examination. The transthoracic echocardiography was standardized using the apical, parasternal, subcostal and, occasionally, the suprasternal approaches (Vingmed Sound System 5, Norway). The echocardiographic criteria of major embolic events included pulmonary hypertension, RV afterload stress and tricuspidal regurgitation. Pathological changes in serial 12-lead electrocardiograms were recorded such as ST-depressions, right precordial T-wave inversions, complete right bundle branch blocks (RBBBs), atrial fibrillation, S1Q3T3 pattern and right axis deviation.
Ventilation/perfusion lung scans were performed in all patients presenting with symptoms suggestive of pulmonary embolism. For ventilation studies, technetium 99m diethylene triamine pentaacetic acid (DTPA) aerosol was used; and for perfusion studies, technetium 99m macroaggregated to albumin was used. V/Q scintigraphy was classified as high, moderate, low or normal/almost normal according to the PIOPED (Prospective Investigation of Pulmonary Embolism Diagnosis) criteria (5).
Diagnosis of PE
A diagnosis of PE was suggested by: a high-probability ventilation-perfusion scan according to PIOPED criteria (5); an indeterminate ventilation-perfusion lung scan and confirmed lower limb deep vein thrombosis on venous ultrasound (6); or the finding of an intraluminal filling defect on PE protocol of contrast-enhanced helical chest computed tomography (7). The detection of an increased D-dimer along with the right ventricle dysfunction (RVD) on transthoracic echocardiography had a high clinical probability for PE in patients for whom multidetector CTPA or V/Q scintigraphy could not be performed (8). According to these criteria, the patients were included in the PE (+) group.
The patients with PE were divided into three groups according to the clinical and hemodynamic features, as follows: massive PE (shock and/or hypotension), sub-massive PE (RVD on TTE, but hemodynamically stable) and non-massive PE.
Statistical analysis
The data obtained from the patients were converted into numeric values and transferred to SPSS 13.0 software. In the analyses of the data, the significance of the difference between two parameters was used for parametric measurements and chi-square test in 2×2 alignments for non parametric measurements. The data was presented as arithmetic median ± standard deviation, the number of the individuals and the percentage. The misapprehension level was accepted as 0.05.
Results
Of 106 patients included in the study, 63 (59%) were PE (+) and 43 (41%) were PE (–) patients. The mean age was 63±15 years. No difference was determined between two groups when some of the demographic features and accompanying diseases were compared (P>0.05) (Table 1).
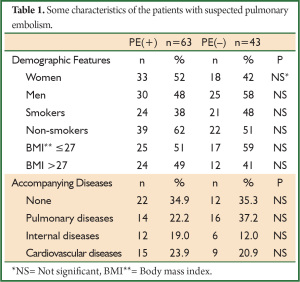
Full table
ECG, CTPA, V/Q scintigraphy, lower extremity venous Doppler US, and TTE were performed in 106 (100%), 90 (84.9%), 75 (70.7%), 93 (87.7%) and 84 (79.2%) of patients with suspected PE, respectively.
In the evaluation of ECG findings, more than half of the PE (–) group (52.8%) had normal ECG whereas only 27.4% of the PE (+) group had normal ECG (P=0.012). S1Q3T3 pattern was more frequently determined in PE (+) group (P<0.05) (Table 2).
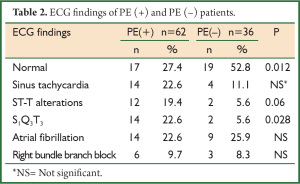
Full table
When transthoracic echocardiography findings were compared, PHT was more commonly determined in PE (+) group (P=0.031) (Table 3).
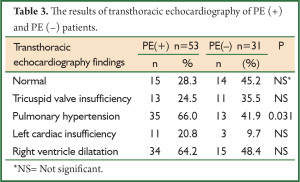
Full table
The cut-off value for the serum cTnI was accepted as 0.01 ng/dL. The values above and those below this limit were accepted as cTnI (+) and cTnI (–), respectively. cTnI positivity was determined in 50.8% (n=32) of the patients with PE and in 11.6% (n=5) of those without PE (P<0.001). It was calculated that high cTnI level had 50.7% sensitivity and 88.3% specificity, positive predictive value (PPV) was 86.4% and negative predictive value (NPV) was 55.8%. The results of cTnI of both groups were presented in Table 4.
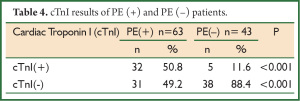
Full table
D-dimer values were measured in 97 patients. When increased D-dimer and cTnI levels were considered together, the sensitivity and the specificity were calculated as 93.5% and 54.5% respectively.
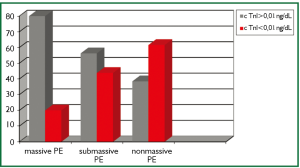
When the PE severity of the patients were classified as massive, sub-massive and non-massive, 7.9% of the patients (n=5) had massive, 50.7% (n=32) had sub-massive and 41.7% (n=26) had non-massive PE. cTnI levels were found to be high in 4 (80%) of 5 patients with massive PE, 18 (56.25%) of 32 patients with sub-massive PE and 10 (38.4%) of 26 patients with non-massive PE (Figure 1).
No statistical relationship was found between the elevated cTnI and ECG findings in PE (+) patients. ECG was normal in 75% of those with cTnI (+); S1Q3T3 pattern was found in the rate of 10%. ST-T changes were found in 9 of 32 patients with high cTnI (28%) and in 3 of 30 patients with normal cTnI (10%) (P=0.071) (Table 5).

Full table
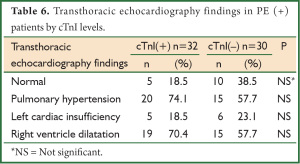
PHT and the right ventricle dilatation were found in the rates of 74.1% and 70.4%, respectively, in PE (+) patients with elevated cardiac troponin I levels whereas both of them were found in the rate of 57.7% in the group with normal cTnI (Table 6).
Full table
Two (3.1%) of the patients accepted as massive emboli from PE (+) group died in the hospital. One of them had RVD findings on TTE and a high cTnI level. The other patient had the signs of right ventricle loading on ECG and high cTnI level; echocardiography could not have been performed, because the patient died in a short time.
When partial arterial oxygen pressure and cTnI levels were analyzed together, no correlation was found in both PE (+) and PE (–) groups.
Discussion
Pulmonary embolus is responsible for 15% of the hospital mortality. The mortality rate of PE reaches up to 30% in patients not treated, this rate declines to 3-10% with prompt diagnosis and accurate treatment. The first step for the diagnosis of PE is the clinical suspicion of PE. The diagnosis of PE is challenging, because clinical signs are nonspecific and laboratory tests can not provide a definitive diagnosis; therefore the delay of the treatment increases the mortality (9-11).
ECG is neither diagnostic nor excluding for the diagnosis of PE. Of the patients with PE, 70% has an abnormality in ECG, but the findings are nonspecific (12). S1Q3T3 pattern, sinus tachycardia and ST-T wave changes can be seen in ECG of the patients with PE. ECG is primarily helpful for the exclusion of such reasons as myocardial infarction and pericarditis (13). In our study, ECG abnormalities were found in 73% of the patients with PE and in half of the patients without PE. S1Q3T3 pattern was more common in PE (+) patients (P=0.028). Our findings are in accordance with the literature.
Particularly in patients with massive PE, right ventricle dilatation, pulmonary artery dilatation and tricuspid regurgitation can be determined by echocardiography (14). Mc Connel et al. found that RVD demonstrated by echocardiography had a specificity of 94% and a sensitivity of 77% for PE diagnosis (15). In our study, transthoracic echocardiography revealed PHT in 35 (66%) of 53 PE (+) patients and right ventricle dilatation in 34 (64.2%) of them. Compared to PE (–) group, PHT was more frequently seen in PE (+) group. These findings suggest that echocardiography is helpful but not diagnostic for PE. The high frequency of PHT and the findings of right ventricle dilatation in PE (–) patients may be related to the accompanying cardiopulmonary diseases in these patients (Table 1). The comparison of previous and current echocardiographic findings of these patients and the evaluation of the changes in pulmonary artery pressure and RVD may be more useful.
In acute PE, an increase in cardiac troponins is seen because of the subendothelial ischemia in the right ventricle (16). The prevalence of elevated cTnI is reported in a variety of rates. In a study, the prevalence of elevated cTnI in patients with PE was found to be 56% (17). Palmieri et al. reported that the prevalence of elevated cTnI in PE was 57% if a cut-off value of 0.1 ng/dL was chosen (18). In another study, cTnI levels and RVD of 36 patients diagnosed with acute PE were investigated and cTnI levels were found to be high in approximately 40% of the patients. Echocardiographic evaluation demonstrated that the most of the patients with high cTnI values developed RVD (19). In our study as well, the prevalence of elevated cTnI was found to be 50.8% in PE (+) group with a cut-off of cTnI of 0.1 ng/dL; and it was significantly higher than PE (–) group. Furthermore, RVD was determined in most of our PE (+) patients with high cTnI (70.4%) and our findings are in accordance with the literature.
The study results showed that combined increase in cTnI and D-dimer had a sensitivity of more than 90% for the diagnosis of PE while the specificity was low, possibly due to the presence of other respiratory and cardiologic conditions leading to high cTnI and D-dimer levels. However, the correlation analysis showed no association between cTnI levels and hypoxia.
Eleveted cardiac troponins in PE were associated with the severity (20) and the prognosis (21) of the disease rather than diagnosis. Amorim et al. found higher cTnI levels in sub-massive PE patients compared with non-massive PE patients (22). Similarly, Kucher et al. found higher cTnI levels in massive PE patients compared with non-massive cases (23). In our study, PE (+) patients were classified as massive (shock and/or hypotension), sub-massive (presence of RVD in echocardiography, but hemodynamically stable) and non-massive PE. Of the patients, 7.9% was evaluated as massive, 50.7% was sub-massive and 41.7% was non-massive. cTnI level was found to be high in 4 of 5 patients with massive PE, 18 (56.25%) of 32 patients with sub-massive PE and 10 (38.4%) of 26 patients with non-massive PE. The number of the patients with high cTnI was higher in massive PE compared with sub-massive and non-massive PE, but it was not found to be statistically significant because of the low number of the patients with massive PE (P>0.05).
Konstantinides et al. found that S1Q3T3 pattern, right bundle branch block and T wave changes in ECG were more frequent in PE patients with high cTnI level compared with the patients with normal cTnI level. Furthermore, they indicated that elevated cTnI alone or along with echocardiographic findings could be used for the evaluation of RVD together with the clinical parameters (24). In our study, the most frequent pathological ECG findings seen in PE patients with high cTnI were S1Q3T3 pattern (31.3%) and ST-T wave alterations (28.1%). However, no significant difference in ECG findings was found between the patients with high and with normal cTnI levels. This situation can be related to the presence of accompanying cardiovascular diseases in PE (–) patients.
Mehta et al. found RVD in 15% of the patients with normal cTnI level and in 67% of those with high cTnI. Moreover, they indicated that cTnI positive patients had higher right ventricle systolic pressure and tended to the development of cardiogenic shock (25). In another study, it was denoted that a high cTnI level and RVD combination was sensitive to predict the increase in hospital complications within the first 14 days (26). In our study, RVD was found in 70.4% of the patients with high cTnI and 57.7% of the patients with normal cTnI. Two (3.1%) of our patients with massive PE died in the hospital. One of them had RVD in echocardiography and a high cTnI level. The other patient who had the findings of right ventricle overloading and high cTnI level died before transthoracic echocardiography. We could not have evaluated the effect of elevated cTnI and RVD on the prognosis because of the short follow up period and the low number of our mortal cases.
In our study, the coronary angiography was performed to none of the patients with high cTnI to demonstrate the absence of coronary artery diseases. However, the cases with high cTnI were evaluated by emergency unit physicians and cardiologist and the diagnosis of acute coronary syndrome was excluded. Though, severe PE should be considered in the differential diagnosis of the patients with unexplainable elevated cTnI and chest pain even who have coronary artery diseases.
In conclusion, cTnI levels can increase in patients with PE and an increased cTnI can suggest severe PE in patients who present with chest pain and in whom acute coronary syndrome is excluded.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology), American Heart Association. Circulation 1996;93:2212-45.
- Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance. Chest 2004;125:1877-84.
- Sheehan P, Vasikaran SD. The evolving clinical role of cardiac troponins and new acute myocardial infarction guidelines: Implications for the clinical laboratory. Clin Biochemist Rev 2001;23:52-65.
- Lualdi JC, Goldhaber SZ. Right ventricular dysfunction after acute pulmonary embolism: pathophysiologic factors, detection, and therapeutic implications. Am Heart J 1995;130:1276-82.
- Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA 1990;263:2753-9.
- Turkstra F, Kuijer PM, van Beek EJ, et al. Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med 1997;126:775-81.
- Wittram C, Maher MM, Yoo AJ, et al. CT angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. Radiographics 2004;24:1219-38.
- British Thoracic Society Standards of Care Committee Pulmonary Embolism Guideline Development Group. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax 2003;58:470-83.
- Hildreth MA, Herndon DN, Parks DH, et al. Evaluation of a caloric requirement formula in burned children treated with early excision. J Trauma 1987;27:188-9.
- Mayo JR, Remy-Jardin M, Müller NL, et al. Pulmonary embolism: prospective comparison of spiral CT with ventilation-perfusion scintigraphy. Radiology 1997;205:447-52.
- Blachere H, Latrabe V, Montaudon M, et al. Pulmonary embolism revealed on helical CT angiography: comparison with ventilation-perfusion radionuclide lung scanning. AJR Am J Roentgenol 2000;174:1041-7.
- Piazza G, Goldhaber SZ. Acute pulmonary embolism; Epidemiology and diagnosis. Circulation 2006;114:e28-32.
- Riedel M. Diagnosing pulmonary embolism. Postgrad Med J 2004;80:309-19.
- Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276-315.
- McConnell MV, Solomon SD, Rayan ME, et al. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol 1996;78:469-73.
- Binder L, Pieske B, Olschewski M, et al. N-terminal pro-brain natriuretic peptide or troponin testing followed by echocardiography for risk stratification of acute pulmonary embolism. Circulation 2005;112:1573-9.
- Hsu JT, Chu CM, Chang ST, et al. Prognostic role of right ventricular dilatation and troponin I elevation in acute pulmonary embolism. Int Heart J 2006;47:775-81.
- Palmieri V, Gallotta G, Rendina D, et al. Troponin I and right ventricular dysfunction for risk assessment in patients with nonmassive pulmonary embolism in the Emergency Department in combination with clinically based risk score. Intern Emerg Med 2008;3:131-8.
- Meyer T, Binder L, Hruska N, et al. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol 2000;36:1632-6.
- Janata K, Holzer M, Laggner AN, et al. Cardiac troponin T in the severity assessment of patients with pulmonary embolism: cohort study. BMJ 2003;326:312-3.
- Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation 2007;116:427-33.
- Amorim S, Dias P, Rodrigues RA, et al. Troponin I as a marker of right ventricular dysfunction and severity of pulmonary embolism. Rev Port Cardiol 2006;25:181-6.
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003;108:2191-4.
- Konstantinides S, Geibel A, Olschewski M, et al. Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation 2002;106:1263-8.
- Mehta NJ, Jani K, Khan IA. Clinical usefulness and prognostic value of elevated cardiac troponin I levels in acute pulmonary embolism. Am Heart J 2003;145:821-5.
- Zhu L, Yang YH, Wu YF, et al. Value of transthoracic echocardiography combined with cardiac troponin I in risk stratification in acute pulmonary thromboembolism. Chin Med J (Engl) 2007;120:17-21.


