Enhanced recovery pathway for thoracic surgery in the UK
Introduction
Advances in thoracic imaging, diagnosis and surgery have meant that more patients can be offered thoracic surgery for both malignant and benign conditions. The operation still represents a significant trauma to the patient and is associated with a slow recovery and return to normal activities. The first national thoracic surgery activity report indicates that the average length of stay in the UK is more than 7 days after lung resection (1). The concept of enhanced recovery (ER) was devised to address these concerns. Specifically, ER refers to a package of perioperative interventions designed to minimise the impact of surgery on patients’ recovery. Implementation reduces postoperative complications and allows an early discharge of the patients (2,3), reducing hospital costs (4). The principle of ER has been demonstrated to work in the context of other surgical specialities (5-8), but has not been used for thoracic surgery. The concept of marginal gain is behind the development of the thoracic surgery ER. Whilst each intervention taken individually might not make a difference, the sum of all of them is likely to translate into significant benefit. With that idea in mind we set out to develop our programme.
Methods
An ER protocol was established at our institution following a review of the best evidence available and by studying other surgical specialties. We re-engineered the perioperative pathway by engaging with every person involved, including the patients themselves. The programme was monitored using specifically-designed patients related outcome measures (PROMs). In addition, a wider multi-disciplinary integrated pathway was introduced, and the process involved working together with general practitioners, community nurses, social services and local chest physicians. Integration between primary, secondary and tertiary care allowed us to have a capillary follow-up even for every patient undergoing thoracic surgery and also facilitated data collection for clinical audit and governance purposes.
Preoperative preparation
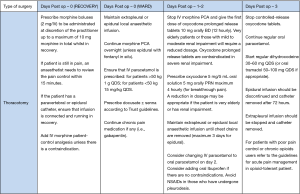
We introduced a “one-stop clinic” strategy to include preoperative investigations, surgical review and consultant-led anaesthetic assessment (9), and this allowed us to start same-day admission. As part of the consent process, patients were informed that they were expected to take an active participation in their care and recovery. We fully embrace the concept of patients being “partners in care” with us.
We believed that patients education was paramount to the success of the project, so we developed a new booklet and DVD disc with detailed information on preoperative exercises, the in-patient pathway and what to expect following discharge, and give this to all patients. This was in addition to the information provided by specialist nurse and surgeons at traditional preoperative consultations. They also receive advice about preoperative fitness (10) and smoking cessation (11-13) where applicable. Each patient’s medications are now analysed by a pharmacist. Patient with chronic obstructive pulmonary disease (COPD) should have their treatment optimised and those patients with a new diagnosis of COPD should be commenced on long-acting inhalers in line with NICE guidance (14-16). Malnourished patients are referred to community dieticians and prescribed nutritional supplements (17). Immobile patients can be referred for occupational therapy aids and social services can be forewarned if support packages are required, a target date for discharge is set to allow patients and their relatives to plan appropriate support. Correction of anaemia (haemoglobin <11 g/dL in women and <12 g/dL in men) should be aimed in all patients (18). The main aim of this clinic is to ensure that patient’s is in the best possible physical condition for surgery and their co-morbidities are fully optimised (19).
Intraoperative management
Adequate hydration facilitates intraoperative fluid management and reduces postoperative pulmonary complications. Hence, patients are asked to fast for only six hours for food and two hours for fluids (20). Premedication is no longer administered routinely, and patients are transferred to the operating theatre in a wheelchair; such intervention facilitates patient mobilisation right up to the time of surgery (21). Antibiotic prophylaxis should be given 60 minutes before the surgery and deep vein thrombosis prophylaxis should be prescribed as per current hospital protocol. Three key elements formed the ER pathway: video assisted thoracoscopic surgery (VATS) (22,23); short-acting anaesthetic drugs and limited invasive monitoring; and aggressive pain management. Central venous access and urinary catheterisation is reserved for patients who are at high-risk of haemodynamic instability or likely to require inotropic support (21).
Postoperative management
In addition to standard care, immediate postoperative recovery focuses on aggressive pain control and antiemetic prophylaxis. Multimodal analgesia is prescribed using the combination of paracetamol, nonsteroidal anti-inflammatory drugs (not if patient underwent a pleurodesis), opioids (morphine patient control analgesia on day 0 and 1, later oral dihydrocodeine and morphine sulphate or oxycodone prolonged release tablets if patient underwent a thoracotomy) and a regional technique [paravertebral/extrapleural, epidural or intercostal block analgesia depending on the surgical approach (24,25)]. A detailed postoperative pain management in case of videothoracoscopic surgery or thoracotomy is described in the Figures 1 and 2. All patients are reviewed by a consultant anaesthetist within 15 minutes if their pain score is above 3/10.

The use of one drain is favourite whenever appropriate, as a recent review concluded that the insertion of one chest drain confers less postoperative pain as shown by a randomized controlled trial (26). Portable suction systems are now employed instead of traditional suction which requires the outlet to be connected to a wall suction system (27,28). This allows immediate mobilisation by physiotherapists and reduced the number of X-rays required. Suction is not routinely recommended. A recent meta-analysis concluded that it is not necessary to use suction in the absence of a clinically important postoperative space and that early drain removal could result in shorter hospital stays (29-31). Suction above 4 KPa is not recommended, as there is no evidence in the literature.
Postoperative patients are seen by physiotherapists twice on day 1, then once or more as required daily thereafter (32-34). The nursing and physiotherapy team ensure that patients are mobilized on the day of surgery or as soon as possible thereafter. For patients who find it difficult to walk, a stationary bicycle is provided to maintain momentum with physiotherapy exercises.
Supplemental oxygen is discontinued once oxygen saturations more than 90% (35). To improve the psychological perception of the disease we ask patients to eat their meals at a table rather than in bed, this is an attempt to replicate home conditions and give a feel of progression toward return to normality.
We have also instituted a daily multidisciplinary ward round where all patients are discussed by a team of surgeons, physiotherapists, nurses, occupational therapists, dieticians and pharmacists.
Discharge
In order to prevent administrative delays, discharge medications are ordered when the chest drains are removed. On discharge, patients are asked to contact the thoracic ward if concerns arise. It has been our experience that the majority of questions can be addressed on the telephone by either nursing or medical staff, thereby preventing a visit to the GP or local hospital.
Results
The ER programme for thoracic surgery was compared with traditional surgical pathway. This analysis was confined to consecutive patients undergoing lung resection for cancer to reduce selection bias. Data were compared using student’s t-test and Fishers exact test. One-hundred and fifty-four ER patients were compared with 171 controls from the year before ER was introduced. Baseline characteristics were similar in both groups. There was an 80% increase in same-day admissions, with a net gain of more than 300 patient bed-days. The ER group had a significantly higher number of procedures performed by VATS (ER 32.9% vs. 9.4%, P=0.0001) and a lower rate of admission to the intensive care unit (ER 5.8% vs. 12.9%, P=0.04). Patients on the ER programme had a significantly reduced postoperative length of stay (mean ER 5.2 vs. 11.7 days, P<0.0001). Patient satisfaction was higher in the ER group after a patient survey. The project resulted in a net saving of £214,000 for the Trust for the 2013/2014 financial year. We were also able to increase the number of patients who underwent thoracic surgery in 2013/2014 by 30% (159 patients) compared with 2012/2013.
Conclusions
The ER pathway is considered standard of care at a growing number of institutions. Our analysis has proven that the ER pathway is a safe perioperative management strategy to reduce the length of hospital stay and the costs after major thoracic surgery, without increasing morbidity or mortality. Patient satisfaction was also increased and the protocol’s multidisciplinary nature connects different medical discipline for an improved patient care.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- The Society for Cardiothoracic Surgery of Great Britain and Ireland. Second National Thoracic Surgery Activity & Outcomes Report. Dendrite Clinical System, London, 2011.
- Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. [PubMed]
- Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189-98. [PubMed]
- Zehr KJ, Dawson PB, Yang SC, et al. Standardized clinical care pathways for major thoracic cases reduce hospital costs. Ann Thorac Surg 1998;66:914-9. [PubMed]
- Debarros M, Steele SR. Perioperative protocols in colorectal surgery. Clin Colon Rectal Surg 2013;26:139-45. [PubMed]
- Di Rollo D, Mohammed A, Rawlinson A, et al. Enhanced recovery protocols in urological surgery: a systematic review. Can J Urol 2015;22:7817-23. [PubMed]
- Hughes MJ, McNally S, Wigmore SJ. Enhanced recovery following liver surgery: a systematic review and meta-analysis. HPB (Oxford) 2014;16:699-706. [PubMed]
- Nelson G, Kalogera E, Dowdy SC. Enhanced recovery pathways in gynecologic oncology. Gynecol Oncol 2014;135:586-94. [PubMed]
- Association of Anaesthetists of Great Britain and Ireland Ireland. Preoperative assessment: the role of the aneasthetist. AAGBI, London, 2001.
- Pouwels S, Fiddelaers J, Teijink JA, et al. Preoperative exercise therapy in lung surgery patients: A systematic review. Respir Med 2015;109:1495-504. [PubMed]
- Turan A, Mascha EJ, Roberman D, et al. Smoking and perioperative outcomes. Anesthesiology 2011;114:837-46. [PubMed]
- Balduyck B, Sardari Nia P, Cogen A, et al. The effect of smoking cessation on quality of life after lung cancer surgery. Eur J Cardiothorac Surg 2011;40:1432-7; discussion 1437-8. [PubMed]
- Mason DP, Subramanian S, Nowicki ER, et al. Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg 2009;88:362-70; discussion 370-1. [PubMed]
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev 2014;3:CD010844. [PubMed]
- O'Reilly J, Jones MM, Parnham J, et al. Guideline Development G. Management of stable chronic obstructive pulmonary disease in primary and secondary care: summary of updated NICE guidance. BMJ 2010;340:c3134. [PubMed]
- Bölükbas S, Eberlein M, Eckhoff J, et al. Short-term effects of inhalative tiotropium/formoterol/budenoside versus tiotropium/formoterol in patients with newly diagnosed chronic obstructive pulmonary disease requiring surgery for lung cancer: a prospective randomized trial. Eur J Cardiothorac Surg 2011;39:995-1000. [PubMed]
- Win T, Ritchie AJ, Wells FC, et al. The incidence and impact of low body mass index on patients with operable lung cancer. Clin Nutr 2007;26:440-3. [PubMed]
- Clevenger B, Mallett SV, Klein AA, et al. Patient blood management to reduce surgical risk. Br J Surg 2015;102:1325-37. [PubMed]
- Knox M, Myers E, Hurley M. The impact of pre-operative assessment clinics on elective surgical case cancellations. Surgeon 2009;7:76-8. [PubMed]
- Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev 2003.CD004423. [PubMed]
- Anaesthesia: care before, during and after anaesthesia: clinical standards. July 2010. NHS Quality Improvement Scotland, 2010.
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [PubMed]
- Klapper J, D'Amico TA. VATS versus open surgery for lung cancer resection: moving toward a minimally invasive approach. J Natl Compr Canc Netw 2015;13:162-4. [PubMed]
- Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418-26. [PubMed]
- Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026-40. [PubMed]
- Dawson AG, Hosmane S. Should you place one or two chest drains in patients undergoing lobectomy? Interact Cardiovasc Thorac Surg 2010;11:178-81. [PubMed]
- Brunelli A, Salati M, Refai M, et al. Evaluation of a new chest tube removal protocol using digital air leak monitoring after lobectomy: a prospective randomised trial. Eur J Cardiothorac Surg 2010;37:56-60. [PubMed]
- Cerfolio RJ, Bryant AS. The benefits of continuous and digital air leak assessment after elective pulmonary resection: a prospective study. Ann Thorac Surg 2008;86:396-401. [PubMed]
- Deng B, Tan QY, Zhao YP, et al. Suction or non-suction to the underwater seal drains following pulmonary operation: meta-analysis of randomised controlled trials. Eur J Cardiothorac Surg 2010;38:210-5. [PubMed]
- Sanni A, Critchley A, Dunning J. Should chest drains be put on suction or not following pulmonary lobectomy? Interact Cardiovasc Thorac Surg 2006;5:275-8. [PubMed]
- Marshall MB, Deeb ME, Bleier JI, et al. Suction vs water seal after pulmonary resection: a randomized prospective study. Chest 2002;121:831-5. [PubMed]
- Novoa N, Ballesteros E, Jiménez MF, et al. Chest physiotherapy revisited: evaluation of its influence on the pulmonary morbidity after pulmonary resection. Eur J Cardiothorac Surg 2011;40:130-4. [PubMed]
- Kaneda H, Saito Y, Okamoto M, et al. Early postoperative mobilization with walking at 4 hours after lobectomy in lung cancer patients. Gen Thorac Cardiovasc Surg 2007;55:493-8. [PubMed]
- Reeve JC, Nicol K, Stiller K, et al. Does physiotherapy reduce the incidence of postoperative pulmonary complications following pulmonary resection via open thoracotomy? A preliminary randomised single-blind clinical trial. Eur J Cardiothorac Surg 2010;37:1158-66. [PubMed]
- Nomori H, Horio H, Suemasu K. Early removal of chest drainage tubes and oxygen support after a lobectomy for lung cancer facilitates earlier recovery of the 6-minute walking distance. Surg Today 2001;31:395-9. [PubMed]