Conventional transbronchial needle aspiration in community practice
Introduction
Transbronchial needle aspiration (TBNA) of the mediastinal lymph nodes was first ever performed by Eduardo Schipetti in 1949; the procedure was indeed performed using rigid instruments. In year 1981, Wang et al. reported its application via flexible bronchoscope commencing a new era in the field of bronchology (1-6). Since then, the utility of TBNA has extended beyond the mediastinal lymphadenopathy and includes endobronchial as well as peripheral lung lesions and sub-mucosal as well as peribronchial processes (7-14).
The procedure of TBNA can be performed using either the anatomical information provided by the roentgenographic studies or an ultrasonic guidance. The former method is referred as conventional TBNA (C-TBNA) while the latter as endobronchial ultrasound guided TBNA (EBUS-TBNA). A linear probe ultrasound is commonly used to perform the TBNA in a real time fashion. Since the introduction of the EBUS-TBNA in 2003 (15), role of C-TBNA is under scrutiny. EBUS-TBNA certainly provides higher diagnostic yield, especially while dealing with the smaller lymph nodes located in the difficult locations such left paratracheal area, yet the procedure has its own limitation. Monitory resources and opportunities to acquire skills at this technique are almost non-existent in the developing world (16). In this context, C-TBNA continues to play a major role in improving the diagnostic yield of flexible bronchoscopy (FB). The procedure is easy to learn and requires zero upfront cost. Any community pulmonologist can acquire and maintain the skills of C-TBNA without undergoing formal interventional pulmonary fellowship training. The following chapter deals with role of C-TBNA in a community practice.
Acquiring skills of C-TBNA
In my personal opinion and experience it is very easy to acquire skills of C-TBNA (17). To begin with, however, every pulmonary fellowship training should offer training in C-TBNA. However if one has missed this opportunity there are several avenues to explore. One can be introduced to C-TBNA at any of the hands-on courses being offered throughout the year at various national and international meetings. One can gather preliminary knowledge related to the indications, anatomical landmarks, role of roentgenography, the instrument, the technique, avoidance of the false positive results, handling of the specimen, required team work and complications of the procedure. Such courses also offer practicing C-TBNA on inanimate objects or even animal model.
Selection of the training program depends upon its reputation, faculty and hours of hands-on sessions. Courses with limited number of attendees allow more opportunity for the one on one discussion with the faculty. Use of animal objects also provides feeling close to performing a real procedure. Some programs also provide training on human cadavers. Live demonstrations are also very useful however such practices are becoming infrequent. Reading material and literature provided at such courses is extremely useful as the pulmonologist starts embarking on the procedure. Several manufacturing companies provide digital videos on C-TBNA which are very useful in studying minor details related to the procedure. Visiting a center of excellence where large number of such procedures is performed might add to the confidence of performing the first procedure. There are certain bronchoscopy centers around the world which may allow real hands on experience to qualified individuals at a cost. If there is a possibility of a preceptor ship from a senior or more experienced colleague in one’s own institution that could be an invaluable resource.
In other words, C-TBNA can be learned “by the books”; post-graduate courses, workshops or hands-on courses (17). Even in the era of endobronchial and the esophageal ultrasounds (EUS), acquiring skills to perform C-TBNA is essential.
Setting up the C-TBNA program
Before commencing the C-TBNA program there are several steps that are crucial. It is mandatory to identify a trained bronchoscopy assistant for the program. He/she should be a fully cognizant of the entire bronchoscopy procedure, cleaning, disinfection and storage of the bronchoscope, types of the needles, specimen preparation and processing. It has very well said by someone that when it comes to bronchoscopy, “you are only as good as your assistant”.
Before sending any specimen to the laboratory a formal meeting with the cytopathologist is essential. This dialogue should include establishing type of preservative to be used, smear techniques, special solutions required for bacterial cultures and lymphoma studies. If there is a possibility, rapid on-site cytological examination (ROSE), that should be certainly explored.
Close collaboration with the thoracic surgeon as well as the radiologist is essential for the success of the program. This would help identify patients who would be best benefitted by the C-TBNA and reduce the need for invasive procedures including cervical mediastinoscopy. It is essential to have a computed tomography (CT) of the chest prior to all elective bronchoscopy procedures. Routinely reviewing the CT scan of the chest of every individual undergoing the procedure with the radiologist, for the accurate location of the lymphnode certainly facilitates the procedure. Such discussions help create a mental image of the aspiration site before entering the bronchoscopy suite.
In these eras of multidisciplinary approach, routine tumor board meetings to discuss the outcomes of individual patients shall increase the academic value of the program.
Rapid on-site evaluation (ROSE) in community practice of TBNA
ROSE of transbronchial aspirates by a cytopathologist present in the bronchoscopy suit reduces the incidence of inadequate specimens and improved C-TBNA yield in observational studies (18,19). There are several plausible reasons why ROSE can improve the yield of TBNA. Firstly, negative or uncertain findings on ROSE can be addressed immediately with repeated aspirations of the same site with a slightly modified technique. This feedback-guided strategy leads to a variable number of aspirates and is indisputably better than sampling an arbitrary number of aspirates of uncertain quality (19). Secondly, the often minute TBNA samples are handled and processed in the best possible way with ROSE, which is an often overlooked but important factor for good TBNA yield (20). Thirdly, the availability of ROSE leads to a more frequent use of C-TBNA and hence practical expertise, which is likely to improve the performance of both bronchoscopists and cytopathologist (21). Furthermore, ROSE encourages the use of TBNA not only for formal staging but also for submucosal, exophytic and peripheral lesions, which are known to have a good yield with C-TBNA (10). ROSE provides the opportunity to stop sampling when the diagnostic objective has been met. Although some trials showed an increase of diagnostic yield ranging from 25% to 46% when ROSE was used along with C-TBNA (18,20), a randomized trial showed no significant difference between the TBNA group and the ROSE group in terms of diagnostic yield (22). It has been shown that the reduced number of samples sent for pathology and microbiology tests as well as the reduced use of consumables that was possible in the ROSE group likely helped abate the costs of bronchoscopy and compensate for the costs of the cytopathology service (23,24).
It is indeed challenging to carry out ROSE in the community practice as expertise of an expert cytopathologist may not be readily available. However such limitation can be overcome by pulmonologist training him/herself in the interpretation of the cytology specimen. In an elegant study by Bonifazi et al., a good concordance was found between the cytopathologist and an adequately trained pulmonologist while dealing with specimens positive with malignant cells (25,26).
Indications
C-TBNA provides tissue specimen for either cytologic or a histological examination from beyond the confines of the endobronchial tree. The size of the specimen thus obtained depends upon the gauge of the needle used, 19 vs. 22 G.
The indications for TBNA are summarized in the table (Table 1). The most common application of TBNA is the diagnosis of mediastinal lymphadenopathy especially in patients suspected to have bronchogenic carcinoma. Conditions such as sarcoidosis, tuberculosis, lymphoma, and post-transplant lymphoproliferative disorders (PTLD) (27) presenting in a similar fashion, can also be diagnosed using C-TBNA. In such cases, the procedure is performed in an elective fashion once the abnormality is detected on the CT scan of the chest. On the other hand, C-TBNA can also be used to sample submucosal as well as peribronchial disease often encountered as an incidental finding during bronchoscopy being performed for other indications. C-TBNA has also been found useful in the diagnosis of peripheral lung lesion especially those with a negative bronchus sign, often referred as Tsuboi type IV lesion (Figure 1). Occasionally C-TBNA is also performed to sample exophytic lesion when excessive bleeding is feared with endobronchial biopsy (EBBx). In experts’ opinion C-TBNA produces much less bleeding than EBBx while dealing with highly vascular lesions. Incidentally, superior vena cava syndrome is not a contraindication to performing TBNA. Similarly C-TBNA is performed in cases of necrotic endobronchial lesion where EBBx is likely to be false negative. In such cases C-TBNA is used to obtain tissue from the core of the lesion, to avoid false negativity (28-30).

Full table
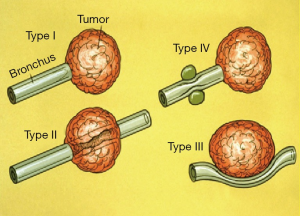
It needs to be mentioned here that C-TBNA is much less sensitive than EBUS-TBNA while performing mediastinal staging for suspected or known bronchogenic carcinoma. Systemic staging of lung cancer (medical mediastinoscopy) is best performed using EBUS-TBNA under general anesthesia, preferably using ROSE by an interventional pulmonologist. C-TBNA could certainly be used to sample large (>15 mm) mediastinal LN preferably to establish unresectable nature of bronchogenic carcinoma.
Results
Diagnosis specific
Staging of non small cell bronchogenic carcinoma
Accurate staging of lung cancer with preoperative detection of mediastinal spread is critical to planning optimal treatment, including resection with curative intent. Lymph node enlargement on CT scan or uptake of fluorodeoxyglucose on positron emission tomography (PET) does not constitute proof of malignant spread. A 2013 meta-analysis reported sensitivity for CT and PET scan of 55% and 77%, respectively (31). In a community practice the use of C-TBNA in staging lung cancer is rare as EBUS-TBNA is superior to C-TBNA and has become the preferred first-step procedure (30,32-34).
The yield of C-TBNA in the diagnosis and staging of lung cancer has been reported between 20% and 80% in the literature (14,35,36). A single meta-analysis found sensitivity and specificity of C-TBNA for the diagnosis of non-small cell lung cancer to be 39% and 99%, respectively (33). Carinal involvement, subcarinal lymph node size greater than 1.5 cm and suspected small cell carcinoma are the factors that increase the sensitivity of C-TBNA (21,37). The diagnostic yield of C-TBNA on small cell lung cancer was reported as high as 89% in the literature (38). It needs to be pointed out here that the sensitivity of C-TBNA depends upon the prevalence of the disease in the cohort being studied (39).
Sarcoidosis
The availability of the 19 G histology needle has expanded the indications for C-TBNA in sarcoidosis; as it is a less invasive, safer, and more economical alternative than mediastinoscopy (Figure 2). Several reports have confirmed the diagnostic value of TBNA for the biopsy of mediastinal lymph nodes in patients with suspected sarcoidosis (40-44). A study involving 258 patients with suspected sarcoidosis found the diagnostic yield of bronchoscopy increased from 66% to 78% percent when C-TBNA was added to the transbronchial biopsy (TBBx) (45). A recent randomized multicenter study of 304 patients with suspected stage I/II sarcoidosis (GRANULOMA) reported that compared to transbronchial biopsy, TBNA of mediastinal nodes by endobronchial ultrasound or EUS resulted in a higher diagnostic yield 80% vs. 53% (43). However, the study should be interpreted with caution given that not all patients with stage I sarcoidosis need histologic diagnosis and not all interventional bronchoscopists are proficient in both procedures. Gupta et al. have showed that, EBUS-TBNA has the highest diagnostic yield in sarcoidosis, but it should be combined with TBBx for the optimal yield. Incidentally the diagnostic yield of C-TBNA plus EBBx and TBBx is similar to EBUS-TBNA plus TBBx (44).
Lymphoma
The clinical utility of C-TBNA in the diagnosis of lymphoma has been limited, since this usually requires larger samples of tissue than are normally obtained by the 22 G needle. However, the availability of the 19 G histology needle, along with the use of flowcytometry to enhance diagnostic yield, may change this assumption (46). The diagnosis of lymphoma, using both cytology and histology needles, has been reported anecdotally, although the overall yield of the procedure in this setting cannot be stated with certainty (27).
Infections
In the literature, a number of anecdotal reports of infections diagnosed via C-TBNA have appeared. C-TBNA can establish the diagnosis of histoplasmosis, pneumocystis jirovecii pneumonia, and cryptococcal infection in patients with AIDS (47). In addition, the diagnosis of mediastinal mycobacterial adenitis (due to either mycobacterium tuberculosis or M. avium-intracellulare) has been described in immunocompetent, as well as immunocompromised patients (48-51). Harkin et al. demonstrated the usefulness of C-TBNA in HIV patients. Forty-one HIV(+) patients with mediastinal lymphadenopathy underwent 44 TBNA procedures. Of 23 procedures performed on patients shown to have mycobacterial disease, aspirations showed smear positive for AFB in 11 (48%), 14 (61%) specimens grew mycobacteria in cultured material, and caseous necrosis or necrotizing granulomatous lesions were seen in 15 (65%). In 48% and 68% of the patients TBNA was exclusive diagnostic for mycobacterial disease in two different studies (52,53).
Miscellaneous
C-TBNA of submucosal area proximal to an endobronchial tumor to detect local spread may help predict the line of surgical resection in patients with non-small-cell carcinoma (54). C-TBNA has also successfully identified leiomyoma of the esophagus (47), metastatic uterine rhabdomyosarcoma (55), sclerosing hemangioma (56), malignant mesothelioma (57), carcinoid tumors (58) and malignant melanoma (59). Mediastinal cysts have also been diagnosed and therapeutically aspirated using the transbronchial route using TBNA needles (60-62). One report described a patient with a right paratracheal mass on CT scan that was suspicious for malignancy; TBNA revealed serosanguineous fluid suggestive of a sterile abscess, and there was no recurrence on later scans following the aspiration (63). Decompression of a subcarinal cyst using TBNA permitted safe anesthesia and subsequent resection of the cyst in another report (64).
Lesion specific
Peripheral nodules or masses
C-TBNA of the peripheral nodules and masses using the 22 G cytology needle has emerged as an extremely useful diagnostic technique. C-TBNA is particularly useful for increasing the diagnostic yield of FB for lesions in which it is not accessible by the common accessories due to the local anatomy and/or its relationship with the adjacent bronchus. Tsuboi type III and IV lesions with negative bronchus sign afford most exclusivity to C-TBNA. A 2013 meta-analysis revealed that the yield of C-TBNA is higher (sensitivity 63%) in peripheral lesions than from either TBBx alone or a combination of TBBx, brushing, and lavage procedures (65). Lesion characteristics predicting the best diagnostic yields are lesions greater than 2 cm in diameter (80% versus 33% to 58%), concurrent mediastinal disease (89% versus 46%), and the lesion being a hematogenous metastasis. The yield from a combination of C-TBNA plus forceps biopsy may be as high as 75%. Although the yield is lower than transthoracic needle aspiration (TTNA), there is a lower risk of pneumothorax (66).
Peribronchial and submucosal disease
The diagnostic yield of conventional procedures such as forceps biopsy and brushing tends to be much lower for submucosal and peribronchial diseases than for the exophytic lesions. Such lesions are often covered by normal epithelium, causing suboptimal sampling. Submucosal infiltration by tumor may make tissues firmer, causing the forceps to slide off the lesion and peribronchial lesions are inaccessible to the biopsy forceps by virtue of being located outside the airway. Under such circumstances C-TBNA to obtain submucosal or peribronchial samples could increase diagnostic yield of FB (9,10,14). In a study involving 31 patients with submucosal and peribronchial disease it was found that the sensitivity of biopsies obtained by forceps, C-TBNA, a combination of both forceps and TBNA, and a combination of forceps biopsy, bronchial brushing and washing, and C-TBNA was 55%, 71%, 89%, and 97%, respectively (9). Thus C-TBNA can significantly increase the diagnostic yield of FB while dealing with peribronchial and submucosal diseases.
Exophytic lesion
Due to the high yield of forceps biopsy for diagnosing endobronchial lesions (67-100%) suspicious for lung cancer, role of TBNA may be limited (67). However, as discussed earlier TBNA has been found exclusively diagnostic while dealing with necrotic or hemorrhagic exophytic lesions (21,68). Overall, reported diagnostic yield of C-TBNA for central lesions suspected to be bronchogenic carcinoma is between 70-96% (10,11,58,67,69).
Complications
Complications of TBNA are uncommon if appropriate precautions are taken and the proper technique is employed. A coagulation profile is not needed prior to TBNA in the absence of a history of a bleeding diathesis (70). Although a variety of complications related to TBNA have been reported, damage to the working channel of the bronchoscope is by far the most important. This complication is more common when a 19 G needle is used, and great care needs to be taken while manipulating the apparatus through the bronchoscope (71). The incidence of fever and bacteremia has been debated, and no firm recommendations can be made regarding antibiotic prophylaxis (72). Transient bacteremia six hours after the procedure with prompt defervescence after antibiotic therapy has been reported. There is a single case report of a purulent pericarditis with polymicrobial mouth flora requiring pericardiocentesis and catheter drainage in addition to antibiotics following a C-TBNA of a mediastinal mass (73). There are no reports of significant bleeding following C-TBNA even in in patient receiving anticoagulation therapy (74). Oozing of a minimal amount of blood from the puncture site may be encountered; the source is usually a dilated blood vessel in the tracheobronchial wall rather than invasion of a major mediastinal vascular structure.
Less frequent complications include pneumothorax, pneumomediastinum, hemomediastinum, and mediastinitis (6,75,76). None of these complications are serious or frequent enough for a community pulmonologist to deter from the valuable procedure of C-TBNA.
Local experience and the learning curve
At our facility possibility of acquiring tools and training required for EBUS-TBNA are close to nil. There are also financial limitations related to the disposable accessories and repair and maintenance of the EBUS scope (77). Beside, our patient volume may not allow adequate number of procedures to maintain proficiency with the EBUS-TBNA. Hence, C-TBNA remains the only option in our environment. Incidentally, there are no interventional pulmonology fellowship programs being offered in our country that provide advanced diagnostic skills. Under the circumstances, we were successful in acquiring the skills of C-TBNA by reading the books and without any formal training. We were also able to achieve proficiency with the procedure in about 60 procedures and have been able to improve the diagnostic yield of bronchoscopy (Figure 3) (78).

Our overall diagnostic yield of C-TBNA since the beginning of the program has been between 41% and 50% (17,78,79). This yield in the patients with lung cancer was found to be 66-82% and 75% in patient with sarcoidosis 75%. Obviously, our yield was higher with lymphnode size greater than 20 mm than otherwise (17,78,79). Understanding of the mediastinal anatomy, refinement of the technique and the preparation of the specimen were the major determinant of the learning curve and the current success of our program. For a novice bronchoscopist, it would be my recommendation to start performing C-TBNA in patients with large mediastinal LNs, located in the favorable location such as subcarinal or right paratracheal area and where small cell carcinoma is a suspect. I would also recommend that one performs at least 25 TBNAs using a cytology needle (22 G) before embarking on the use of a histology needle (19 G). The latter may require added skill and experience for its insertion through the tracheobronchial wall and to avoid damage to the vascular structures (17,78). It does need to be pointed out that in our part of the world, patients seldom present with resectable lung cancer and even patients with benign conditions exhibit bulky lymphnodes where use of C-TBNA is most appropriate.
Conclusions
TBNA provides an opportunity to diagnose mediastinal lesions and stage bronchogenic carcinoma in a minimally invasive fashion. In our opinion acquiring skills of C-TBNA doesn’t require formal interventional pulmonology training and could be learned from the “books”. Proficiency could be attained within 60 procedures and acceptable results could be achieved. There is no upfront cost and the procedure can be performed under moderate sedation and local anesthesia in a cost effective fashion. If a possibility exists, a bronchoscopist can also train him/herself with interpretation of cytology specimen for ROSE. The procedure is safe and has great potential to augment the welfare of patients with pulmonary ailment. Every community pulmonologist should be able to perform C-TBNA.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Schieppati E. La puncion mediastinal a traves del espolon traqueal. Rev As Med Argent 1949;63:497. [PubMed]
- Brouet G, Paley PY, Marche J, et al. Puncture for cytodiagnosis of peri-tracheo-bronchial isolated adenopathy. J Fr Med Chir Thorac 1953;7:393-8. [PubMed]
- Euler HE, Strauch J, Witte S. Cytodiagnosis of mediastinal tumors. Arch Ohren Nasen Kehlkopfheilkd 1955;167:376-82. [PubMed]
- Schieppati E. Mediastinal lymph node punctures through the tracheal carina. Surg Gynecol Obstet 1958;107:243-6. [PubMed]
- Schiessle W. Transbronchial and transtracheal puncture in peritracheobronchial adenopathies. J Fr Med Chir Thorac 1962;16:551-69. [PubMed]
- Wang KP, Marsh BR, Summer WR, et al. Transbronchial needle aspiration for diagnosis of lung cancer. Chest 1981;80:48-50. [PubMed]
- Wang KP, Terry PB. Transbronchial needle aspiration in the diagnosis and staging of bronchogenic carcinoma. Am Rev Respir Dis 1983;127:344-7. [PubMed]
- Shure D, Fedullo PF. Transbronchial needle aspiration of peripheral masses. Am Rev Respir Dis 1983;128:1090-2. [PubMed]
- Shure D, Fedullo PF. Transbronchial needle aspiration in the diagnosis of submucosal and peribronchial bronchogenic carcinoma. Chest 1985;88:49-51. [PubMed]
- Dasgupta A, Jain P, Minai OA, et al. Utility of transbronchial needle aspiration in the diagnosis of endobronchial lesions. Chest 1999;115:1237-41. [PubMed]
- Hermens FH, Van Engelenburg TC, Visser FJ, et al. Diagnostic yield of transbronchial histology needle aspiration in patients with mediastinal lymph node enlargement. Respiration 2003;70:631-5. [PubMed]
- Sharafkhaneh A, Baaklini W, Gorin AB, et al. Yield of transbronchial needle aspiration in diagnosis of mediastinal lesions. Chest 2003;124:2131-5. [PubMed]
- Khoo KL, Chua GS, Mukhopadhyay A, et al. Transbronchial needle aspiration: initial experience in routine diagnostic bronchoscopy. Respir Med 2003;97:1200-4. [PubMed]
- Hsu LH, Liu CC, Ko JS. Education and experience improve the performance of transbronchial needle aspiration: a learning curve at a cancer center. Chest 2004;125:532-40. [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [PubMed]
- Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6. [PubMed]
- Kupeli E, Memis L, Ozdemirel TS, et al. Transbronchial needle aspiration "by the books". Ann Thorac Med 2011;6:85-90. [PubMed]
- Diette GB, White P, Terry P, et al. Utility of on-site cytopathology assessment for bronchoscopicevaluation of lung masses and adenopathy. Chest 2000;117:1186-90. [PubMed]
- Chin R, McCain TW, Lucia MA, et al. Transbronchial needle aspiration in diagnosing and staging lung cancer: How many aspirates are needed? Am J Respir Crit Care Med 2002;166:377-81. [PubMed]
- Davenport RD. Rapid on-site evaluation of transbronchial aspirates. Chest 1990;98:59-61. [PubMed]
- Haponik EF, Cappellari JO, Chin R, et al. Education and experience improve transbronchial needle aspiration performance. Am J Respir Crit Care Med 1995;151:1998-2002. [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Rapid on-site evaluation of transbronchial aspirates in the diagnosis of hilar and mediastinal adenopathy: a randomized trial. Chest 2011;139:395-401. [PubMed]
- Baram D, Garcia RB, Richman PS. Impact of rapid on-site cytologic evaluation during transbronchial needle aspiration. Chest 2005;128:869-75. [PubMed]
- Diacon AH, Schuurmans MM, Theron J, et al. Utility of rapid on-site evaluation of transbronchial needle aspirates. Respiration 2005;72:182-8. [PubMed]
- Bonifazi M, Sediari M, Ferretti M, et al. The role of the pulmonologist in rapid on-site cytologic evaluation of transbronchial needle aspiration: a prospective study. Chest 2014;145:60-5. [PubMed]
- Mehta AC, Cicenia J. ROSEs Are Read. Chest 2014;145:7-9. [PubMed]
- Ghamande S, Rafanan A, Dweik R, et al. Role of transbronchial needle aspiration in patients receiving mechanical ventilation. Chest 2002;122:985-9. [PubMed]
- Ernst A, Silvestri GA, Johnstone D. American College of Chest Physicians. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. [PubMed]
- Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002;19:356-73. [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Herth F, Becker HD, Ernst A. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest 2004;125:322-5. [PubMed]
- Holty JE, Kuschner WG, Gould MK. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax 2005;60:949-55. [PubMed]
- Navani N, Nankivell M, Lawrence DR, et al. Lung-BOOST trial investigators. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med 2015;3:282-9. [PubMed]
- Schenk DA, Bower JH, Bryan CL, et al. Transbronchial needle aspiration staging of bronchogenic carcinoma. Am Rev Respir Dis 1986;134:146-8. [PubMed]
- Toloza EM, Harpole L, Detterbeck F, et al. Invasivestaging of non-small cell lung cancer: A review of the currentevidence. Chest 2003;123:157S-166S. [PubMed]
- Harrow E, Halber M, Hardy S, et al. Bronchoscopic and roentgenographic correlates of a positive transbronchial needle aspiration in the staging of lung cancer. Chest 1991;100:1592-6. [PubMed]
- Caglayan B, Akturk UA, Fidan A, et al. Transbronchial needle aspiration in the diagnosis of endobronchial malignant lesions: a 3-year experience. Chest 2005;128:704-8. [PubMed]
- Medford AR, Agrawal S, Free CM, et al. A prospective study of conventional transbronchial needle aspiration: performance and cost utility. Respiration 2010;79:482-9. [PubMed]
- Trisolini R, Lazzari Agli L, Cancellieri A, et al. The value of flexible transbronchial needle aspiration in the diagnosis of stage I sarcoidosis. Chest 2003;124:2126-30. [PubMed]
- Bilaçeroğlu S, Perim K, Günel O, et al. Combining transbronchial aspiration with endobronchial and transbronchial biopsy in sarcoidosis. Monaldi Arch Chest Dis 1999;54:217-23. [PubMed]
- Morales CF, Patefield AJ, Strollo PJ Jr, et al. Flexible transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest 1994;106:709-11. [PubMed]
- von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA 2013;309:2457-64. [PubMed]
- Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest 2014;146:547-56. [PubMed]
- Pauli G, Pelletier A, Bohner C, et al. Transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest 1984;85:482-4. [PubMed]
- Ketai L, Chauncey J, Duque R. Combination of flow cytometry and transbronchial needle aspiration in the diagnosis of mediastinal lymphoma. Chest 1985;88:936. [PubMed]
- Wang KP. Transbronchial needle aspiration to obtain histology specimen. J Bronchol 1994;1:116-22.
- Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest 2004;125:712-22. [PubMed]
- Malabonga VM, Basti J, Kamholz SL. Utility of bronchoscopic sampling techniques for cryptococcal disease in AIDS. Chest 1991;99:370-2. [PubMed]
- Baron KM, Aranda CP. Diagnosis of mediastinal mycobacterial lymphadenopathy by transbronchial needle aspiration. Chest 1991;100:1723-4. [PubMed]
- Calpe JL, Chiner E, Larramendi CH. Endobronchial tuberculosis in HIV-infected patients. AIDS 1995;9:1159-64. [PubMed]
- Harkin TJ, Ciotoli C, Addrizzo-Harris DJ, et al. Transbronchial needle aspiration (TBNA) in patients infected with HIV. Am J Respir Crit Care Med 1998;157:1913-8. [PubMed]
- Bilaçeroğlu S, Günel O, Eriş N, et al. Transbronchial needle aspiration in diagnosing intrathoracic tuberculous lymphadenitis. Chest 2004;126:259-67. [PubMed]
- York EL, Jones RL, King EG, et al. The value of submucous needle aspiration in the prediction of surgical resection line of bronchogenic carcinoma. Chest 1991;100:1028-9. [PubMed]
- Goldstein LS, Kavuru MS, Meli Y, et al. Uterine rhabdomyosarcoma metastatic to mediastinal lymph nodes: diagnosis by transbronchial needle aspiration. South Med J 1999;92:84-7. [PubMed]
- Hirano H, Miyagawa Y, Nagata N, et al. Transbronchial needle aspiration in the diagnosis of pulmonary sclerosing haemangioma. Respir Med 1993;87:475-7. [PubMed]
- Selcuk ZT, Hafiz MA, Wang KP. Malignant pleural mesothelioma diagnosed by transbronchial needle biopsy. J Bronchol 1997;4:136-8.
- Conley YD, Cafoncelli AR, Khan JH, et al. Bronchial carcinoid tumor: experience over 20 years. Am Surg 1992;58:670-2. [PubMed]
- Das RK, Dasgupta A, Tewari S, et al. Malignant melanoma of the bronchus. J Bronchol 1998;5:59-60.
- Scatarige JC, Wang KP, Siegelman SS. Transbronchial needle aspiration biopsy of the mediastinum. In: Siegelman SS, editor. Contemporary Issues in Computed Tomography: Computed Tomography of the Chest. New York: Churchill Livingstone, 1984:59.
- Schwartz AR, Fishman EK, Wang KP. Diagnosis and treatment of a bronchogenic cyst using transbronchial needle aspiration. Thorax 1986;41:326-7. [PubMed]
- Schwartz DB, Beals TF, Wimbish KJ, et al. Transbronchial fine needle aspiration of bronchogenic cysts. Chest 1985;88:573-5. [PubMed]
- Wang KP, Nelson S, Scatarige J, et al. Transbronchial needle aspiration of a mediastinal mass: therapeutic implications. Thorax 1983;38:556-7. [PubMed]
- McDougall JC, Fromme GA. Transcarinal aspiration of a mediastinal cyst to facilitate anesthetic management. Chest 1990;97:1490-2. [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Gasparini S, Ferretti M, Secchi EB, et al. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses. Experience with 1,027 consecutive cases. Chest 1995;108:131-7. [PubMed]
- Popovich J Jr, Kvale PA, Eichenhorn MS, et al. Diagnostic accuracy of multiple biopsies from flexible fiberoptic bronchoscopy. A comparison of central versus peripheral carcinoma. Am Rev Respir Dis 1982;125:521-3. [PubMed]
- Cetinkaya E, Yildiz P, Altin S, et al. Diagnostic value of transbronchial needle aspiration by Wang 22-gauge cytology needle in intrathoracic lymphadenopathy. Chest 2004;125:527-31. [PubMed]
- Jones DF, Chin R Jr, Cappellari JO, et al. Endobronchial needle aspiration in the diagnosis of small-cell carcinoma. Chest 1994;105:1151-4. [PubMed]
- Dasgupta A, Mehta AC. Transbronchial needle aspiration. An underused diagnostic technique. Clin Chest Med 1999;20:39-51. [PubMed]
- Sherling BE. Complication with a transbronchial histology needle. Chest 1990;98:783. [PubMed]
- Witte MC, Opal SM, Gilbert JG, et al. Incidence of fever and bacteremia following transbronchial needle aspiration. Chest 1986;89:85-7. [PubMed]
- Epstein SK, Winslow CJ, Brecher SM, et al. Polymicrobial bacterial pericarditis after transbronchial needle aspiration. Case report with an investigation on the risk of bacterial contamination during fiberoptic bronchoscopy. Am Rev Respir Dis 1992;146:523-5. [PubMed]
- Harrow EM, Oldenburg FA Jr, Lingenfelter MS, et al. Transbronchial needle aspiration in clinical practice. A five-year experience. Chest 1989;96:1268-72. [PubMed]
- Kucera RF, Wolfe GK, Perry ME. Hemomediastinum after transbronchial needle aspiration. Chest 1986;90:466. [PubMed]
- Gochi F, Chen F, Aoyama A, et al. Mediastinal infectious complication after endobronchial ultrasound-guided transbronchial needle aspiration. Interact Cardiovasc Thorac Surg 2013;17:751-2. [PubMed]
- Stather DR, MacEachern P, Chee A, et al. Evaluation of clinical endobronchial ultrasound skills following clinical versus simulation training. Respirology 2012;17:291-9. [PubMed]
- Küpeli E, Seyfettin P, Tepeoğlu MD. Conventional transbronchial needle aspiration: From acquisition to precision. Ann Thorac Med 2015;10:50-4. [PubMed]
- Küpeli E, Cörüt R, Memiş L, et al. Transbronchial needle aspiration: a tool for a community bronchoscopist. J Bronchology Interv Pulmonol 2012;19:115-20. [PubMed]


