Echocardiographic assessment for ventricular assist device placement
Introduction
Left ventricular assist devices (LVAD) have demonstrated considerable benefits for the management of patients with refractory heart failure compared to pharmacotherapy (1,2). Immediate and long-term outcomes of LVAD therapy are dependent on multiple factors including patient selection and optimization, intraoperative management (surgical and anesthetic) and postoperative management such as anticoagulation therapy. Despite these factors, one important aspect of patient management with a direct impact on LVAD outcomes has been the utility of comprehensive intraoperative transesophageal echocardiographic monitoring (3,4).
We provide an overview and discussion of the role of TEE monitoring for LVAD implantation during the operative period. The layout of the review follows the time course one would normally encounter in the operating room with a pre-implantation assessment, immediate post-implantation—expected findings, and post-implantation—unexpected findings.
Pre-implantation assessment
The echocardiographic examination for LVAD placement is multifaceted and complex. The American Society of Echocardiography (ASE) in conjunction with the Society of Cardiovascular Anesthesiologists (SCA) have previously released guidelines for the comprehensive intraoperative echocardiographic assessment of a patient undergoing cardiac surgery (5). Additionally, the recently released ASE guidelines for echocardiographic assessment in the management of patients with an LVAD (including intraoperative LVAD implantation) recommend that the exam be performed by either a cardiologist with significant LVAD and advanced perioperative transesophageal echocardiography (TEE) experience or a cardiothoracic anesthesiologist with advanced perioperative TEE expertise (6).
One should begin with a standard comprehensive TEE exam to confirm preexisting findings especially if these pre-operative exams performed were exclusively transthoracic echocardiograms. Furthermore, the comprehensive TEE examination, despite alterations in hemodynamic physiology under anesthesia, may help recognize any missed diagnoses in cardiac pathology, or significant changes in cardiac function since the prior echocardiographic exam, ultimately affecting the initial surgical plan.
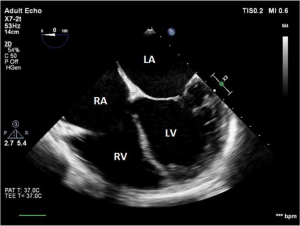
The comprehensive TEE examination should pay particular attention to the confirmation of the primary diagnosis of end-stage left ventricular failure. This is extremely important because the presence of echocardiographic signs demonstrating a significant improvement in left ventricle (LV) systolic function might preclude the placement of a LVAD. The echocardiographic signs of end-stage LV failure include the following, but are not limited to a severely dilated and spherical-shaped LV, the presence of severe segmental wall motion abnormalities, and the presence of spontaneous echo contrast in the LV, concordant with signs of left atrial hypertension (Figure 1). While ejection fraction per se is not important in this context, as end-stage heart failure prognosis is multifactorial (7), it is ideal to obtain a baseline quantification of LV function and LV end diastolic diameter (LVEDD) prior to LVAD implantation. This allows for comparison of geometric size resulting from LV pressure and volume decompression once the LVAD is operational. One should also note the position of the septum, both atrial and ventricular, signifying bowing to the right as a result of increased left atrial pressure (LAP) or increased LV pressure, respectively. Any unexpected findings that could alter the surgical plan [e.g., intracardiac thrombus, severe tricuspid regurgitation (TR)] should be communicated promptly to the surgical team.
In addition to the comprehensive TEE exam, the LVAD specific TEE exam includes several components. Initially, it may be advisable to follow a checklist such as that found in the recently released ASE guidelines for patients with an LVAD (6). While there are several important findings to rule out or confirm, in the opinion of the authors, four are paramount and are denoted by the mnemonic “STAR”: Shunts, Thrombi, Aortic insufficiency (AI) and Right ventricular function.
Intracardiac shunts
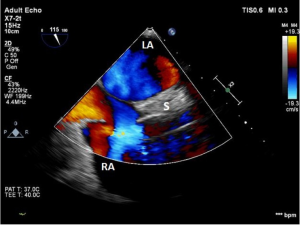
Intracardiac shunts are potentially serious impediments to successful LVAD support. The LVAD, once initiated, can significantly reduce left sided intracardiac pressures to potentially lower pressures in comparison to the right heart, rendering the patient vulnerable to significant hypoxemia if a right to left shunt pathway exists. While any sort of atrial septal defect or ventricular septal defect can be the pathological culprit, by far the most common is a patent foramen ovale (PFO).
Traditional echocardiographic screening methods for a PFO include the use of color flow Doppler across the interatrial septum (at a low Nyquist limit), or the use of contrast echocardiography (injection of agitated saline intravenously). A color flow Doppler interrogation of the interatrial septum can potentially show not only flow communicating between the atria, but also the directionality of the flow (Figure 2). In the operating room during mechanical ventilation, a modified Valsalva maneuver (held positive pressure) is released just as agitated saline, injected via central venous access, is opacifying the right atrium. This can be successful if the atrial septum is seen bowing to the left during the study. Unfortunately, both of these methods may fail to correctly diagnose a PFO, even if performed correctly, in the setting of elevated LAP as a consequence of severe LV failure. In an effort to increase the preimplantation detection of a PFO, some echocardiographers have advocated injecting agitated saline into a femoral vein (6). Another reported method is the occlusion of the main pulmonary artery (PA) just before initiating cardiopulmonary bypass (CPB) in order to reliably create a right to left pressure gradient. However, caution is advised and a rescue strategy recommended if the Valsalva maneuver or PA occlusion methods are to be employed, as these patients often have minimal cardiovascular reserve.
Intracardiac thrombus
An intracardiac thrombus can have devastating consequences, particularly if present in the left atrium or the LV. In particular, proper echo windows should be interrogated in order to visualize, and rule out thrombus in the LV apex and the left atrial appendage. The latter is more likely to contain thrombus if the patient has been in atrial fibrillation, but given the chronic low flow state of these patients, the LV may be just as susceptible to thrombus formation. In both these locations, 3D echocardiographic capability may provide additional clarity. For example, the atrial appendage can be acquired in 3D and then rotated to an “en face” view, enabling the examiner to “look” straight down into the appendage. In ruling out an LV apical thrombus, the examiner should understand the limitations of TEE in viewing the LV apex, compared to transthoracic echocardiography (TTE), considering the LV apex is further (far field) from the position of the transducer in the esophagus. Further, if suspicion for thrombus is high such as in an LV aneurysm, consideration should be given to the use of a microbubble contrast agent to better delineate the possible presence of thrombus. Additionally, 3D echocardiography can also be valuable when assessing the LV free wall (aneurysmal wall) and the apex for thrombus.
Aortic insufficiency (AI)
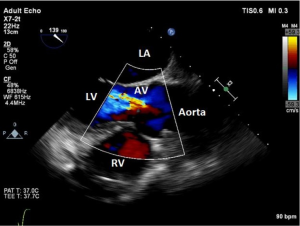
Another significant concern prior to LVAD implantation is the presence of AI. Upon LVAD activation and in the presence of AI, forward systemic flow resulting from the LVAD will be reduced by the regurgitant flow through the incompetent aortic valve back into the LV. In essence, AI produces a circular and futile circulation competing with the forward systemic flow, with the degree of AI directly affecting the overall forward systemic flow. Therefore, it is imperative that the aortic valve should be interrogated from multiple echocardiographic views to appreciate the presence and severity of AI. The aortic valve is best visualized in the mid-esophageal long axis (ME AV LAX), mid-esophageal aortic valve short-axis (ME AV SAX), and the deep transgastric (deep TG) views. The degree of AI is best assessed by utilizing objective measurements such as the vena contracta width of the AI jet, pulsed-wave (PW) Doppler of diastolic blood flow in the descending aorta, ratio of the AI jet width to the left ventricular outflow tract (LVOT) width, and calculation of the regurgitant volume (Figure 3) (8). Severity of AI graded as moderate or greater may require additional surgical intervention (surgical repair or replacement of the aortic valve). It is important to realize that heart failure patients, particularly those under general anesthesia, may have a combination of high LV diastolic pressure and low systemic pressure. This provides a much smaller diastolic gradient across the aortic valve than with LVAD activation when the LV is unloaded and cardiac output is augmented. Thus, it is advisable to augment the systemic blood pressure when evaluating the presence and severity of AI prior to implantation to gain a more appreciable assessment of the valve. Additionally, inspection of the severity of AI should also be performed shortly after going on CPB, as this may create a more significant gradient across the valve as a result of decompressing the LV.
Generally speaking, aortic stenosis (AS) is not a serious impediment to successful LVAD implantation, although the presence of AI typically coincides with AS. If a prosthetic valve is in situ, it should be examined for function and to rule out vegetations or thrombus. Mechanical aortic valve prostheses are considered too thrombogenic for LVAD patients. As such, they are considered a relative contraindication for LVAD implantation, and would likely need to be replaced if such support is contemplated. A similar concern would exist for a mechanical pulmonic valve if RVAD implantation were being considered.
Right ventricular failure
A significant number of patients requiring LVAD therapy have at least some degree of existing right ventricular failure due to elevated pulmonary vascular resistance as a consequence of LV dysfunction (9). Failure of the RV has been reported to occur in up to 44% of LVAD recipients post-implant (9). While recent data with continuous flow pumps such as the HeartMate II (Thoratec, Pleasanton, CA, USA) have demonstrated RV failure rates as low as 5% in some cases, RV failure post LVAD implantation remains a significant problem, and is a significant predictor of morbidity and mortality (10-12).
Several investigators have endeavored to stratify the risks of RV failure in patients presenting for LVAD placement (9,12). These analyses have come up with an assortment of clinical predictors of RV failure (e.g., low PA pressures, renal or hepatic insufficiency, preoperative inotropic support, destination therapy, etc.) (9,12,13).
Preliminary studies have identified some possible echocardiography-based predictors of RV failure. To date, these have been observational studies. The authors are not aware of prospectively validated echocardiographic screening parameters being used to change the therapeutic plan. Nevertheless, one study evaluating RV failure risk found that the only echo parameter predictive of RV failure was “severe” RV systolic dysfunction prior to LVAD placement (as defined by the echocardiographer) (12). More recently, at least two investigators have found positive predictive value for RV failure post implant with RV tissue Doppler velocities (S’ <4.4 cm/s, E/e’ <10) and RV free wall strain (<-14%) (14,15). However, these studies and many others have been performed using transthoracic echocardiography in awake, nonventilated patients.
Aside from predictive efforts, a baseline assessment of the RV should be undertaken. This will allow for predictive assessment of RV function after LVAD implantation and consideration for RV management by means of mechanical or pharmacologic support. Preparation should be taken for means of supporting RV function during and after LVAD implantation. Unlike the ellipse-shaped LV, the shape of the RV is complex and U-shaped. Therefore, multiple echocardiographic views, angles and parameters should be used to best assess RV function (6). The best views for examination of the RV using TEE include the mid-esophageal four-chamber (ME 4C), the mid-esophageal RV inflow-outflow (ME RV inflow-outflow), the trans-gastric RV inflow (TG RV inflow), and the trans-gastric short axis (TG SAX) (Figures 1,4). One should note the size of the RV at end-diastole and whether it is contributing to the formation of the cardiac apex, which indicates RV dilation and potential for RV failure from increased preload once the LVAD is operational. Pre-LVAD fractional area change (FAC), a surrogate for ejection fraction can be a useful index of RV function (16). The tricuspid annular plane systolic excursion (TAPSE) is a reproducible measure of baseline function. Finally, if strain measurement capability is available, consideration should be given to obtaining baseline RV free wall longitudinal strain values, as reduced strain (−9.0 or less) has been associated with increased risk of RV failure after LVAD placement (17).
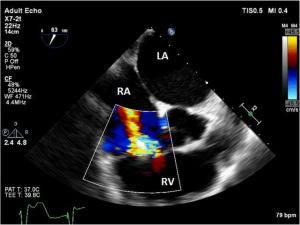
The tricuspid valve, as it is related to RV function, is best viewed on the TEE using the ME 4C, ME RV inflow-outflow, and TG RV inflow views (Figures 1,4). Significant pre-existing TR has been associated with RV failure post-LVAD implantation (18). TR is commonly exhibited as a consequence of progressive RV enlargement. Both the degree and mechanism (organic vs. functional) of TR need to be thoroughly examined, as palliation of moderate or greater TR may improve RV function post LVAD implantation (18). This baseline information is important even in the absence of repair, as post-LVAD TR can be compared to it.
Post-implantation assessment: findings and complications
The use of TEE is invaluable for evaluating both LVAD placement and function post-implantation in the operating room. CPB is commonly used for LVAD insertion; however, device implantation without the use of CPB has been reported (19). Major considerations for the echocardiographic assessment post-implantation include adequate de-airing of the LV and the device, the position of the inflow and outflow cannulas, Doppler velocities of the inflow and outflow cannulas, ventricular volumes and function, native valvular function, and the presence of intracardiac shunts previously undetected during the pre-implant phase.
Intracardiac air
Despite precautions, air commonly becomes trapped in the device and in the LV from coring the apex, and is entrained into the patient’s circulatory system mostly collecting in the LV. TEE is able to rapidly and easily detect residual air in the heart or great vessels throughout the procedure. It is preferable to evacuate air prior to full activation of the device; however, its presence should be expected and minimized prior to release of the outflow cross-clamp and prior to separation from CPB (6). These two periods provide the best opportunity for de-airing while minimizing the risk of complications associated with an air embolism.
Air tends to accumulate in the non-dependent (generally anterior) portions of the LV and left atrium and is visualized as hyperechoic, white speckles on TEE. The mid-esophageal long axis view (ME LAX) provides a good perspective on the presence of air along the anteroseptal wall of the LV resulting either from reestablishing pulmonary perfusion or placement of the VAD itself. The ME 4C view is useful for visualizing residual air along the inferoseptal wall of the LV (20). A thorough evaluation of all windows of the LV should be conducted as well as the LVAD outflow cannula, and thoracic aorta (ascending and descending) to ensure the resolution of air by the de-airing process.
LVAD cannula positioning
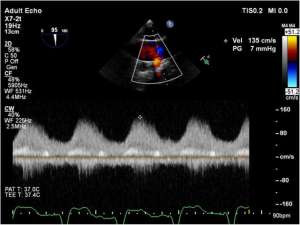
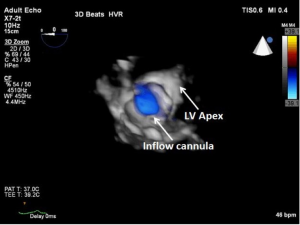
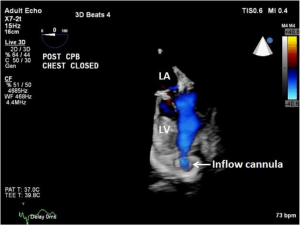
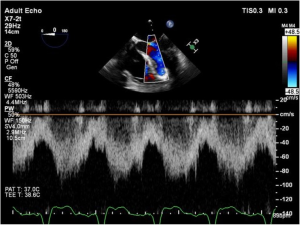
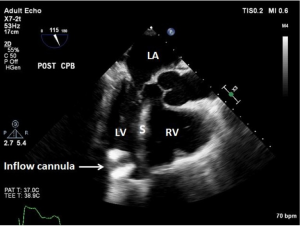
As discussed earlier, the inflow cannula is placed surgically in the apex of the LV. Blood from the LV then flows through the inflow cannula and into the device, which ejects it through the outflow graft into the ascending aorta. The ME 4C view and the ME 2C view can help determine if the cannula is properly aligned with the LV inflow tract (mitral valve opening) and not opposed by the potentially impinging LV septal or lateral walls (Figures 5-7). This allows for optimal laminar flow of blood into the device. A color flow Doppler window should be placed at the inflow cannula site to demonstrate laminar blood flow through the cannula (Figures 8-10). This interrogation of the inflow cannula should reveal velocities ≤1.5 m/s (Figure 11) (6). The measurement of inflow cannula velocities >1.5 m/s require extensive interrogation of the cannula insertion site preferably with 3D echocardiography to rule out potential tissue obstruction (Figure 12) It is important to stress that the inflow cannula position should be evaluated in both views to establish a three dimensional assessment of proper alignment. Recently, the use of 3D imaging has proven successful in assessing inflow cannula alignment as demonstrated in Figures 5,10 (21).
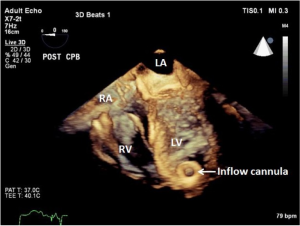

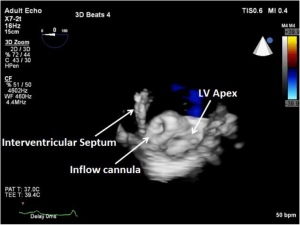
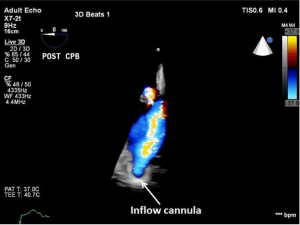

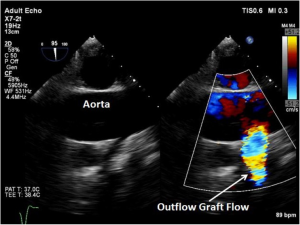
The outflow graft anastomosis to the ascending aorta can be visualized approximately at the level of the right PA. This is best seen in the ME ascending aorta short axis or long axis views. Although, outflow-graft velocity benchmarks are currently not available, color flow Doppler echocardiography can help ensure laminar flow through the outflow graft into the lumen of the ascending aorta from the device (Figure 13). Evidence of high velocities (>2.0 m/s) can be indicative of obstruction of the outflow graft (Figure 14) (6). However, atheroma at the site of the outflow cannula can also lead to turbulent flow as can an outflow cannula placed orthogonally to the insertion site. Placing the outflow cannula at a shallower angle, can improve forward blood flow and reduce turbulence (22). The presence of air bubbles in the ascending aorta near the outflow graft insertion site can be indicative of poor anastomosis, perforation of the cannula or inadequate de-airing. Flow velocities should range from 1.0 to 2.0 m/s for adequate flow (20).
Native cardiac structures
Aside from the device hardware, echocardiographic assessment of the ventricles and native valves should be undertaken once the LVAD is implanted and activated. The ME 4C and TG 2-chamber views can offer the best assessment of the LV, RV, and the interventricular septum. The cardiac valves and great vessels should also be examined to ensure minimal change in valvular function and to rule out iatrogenic complications (i.e., aortic dissection). Since the LVAD flow is continuous and the LV is unloaded, often the degree of mitral regurgitation is lessened. If a concomitant procedure took place during CPB, such as aortic valve replacement, care should be taken to ensure appropriate valve function. As the LVAD speed is slowly and incrementally increased, frequent examination of these cardiac structures will allow optimal management with intravenous fluids and vasoactive infusions. If the intracardiac structures and hemodynamics are not frequently monitored, complications can ensue and are discussed in the following section.
Left ventricular assist devices (LVAD related aortic insufficiency (AI)
Thorough examination of the aortic valve is imperative post-LVAD implantation. Undiagnosed AI can lead to chronic volume overload of the LV leading to further ventricular dilation and worsening heart failure. Under normal conditions, the degree of AI is best evaluated by using multiple TEE views (ME AV SAX, ME AV LAX, and the deep TG LAX views), color M-mode, color flow Doppler (vena contracta), spectral flow Doppler, and pressure half-time (Figure 3). However, after LVAD implantation the diastolic dependent techniques, pressure half-time and PW Doppler are unreliable due to the continuous-flow nature of the device (6). This is due to AI duration extending into the systolic phase.
Uncorrected AI can lead to ineffective LVAD output as the blood recirculates through the LV as already described. This results in poor systemic cardiac output, inadequate oxygen delivery, and end organ malperfusion. Consensus management currently suggests that patients with greater than mild AI undergo either valve replacement or repair (23). Any aortic valve intervention adds to the complexity of the surgery, necessitates additional time on CPB, and can potentially increase procedure-related morbidity (24). The expected duration and indication of LVAD support can influence whether the valve needs correction, replacement or can be left alone. If heart transplantation is eminent or only temporary support is needed (bridge to decision-making), AI may be tolerated with little sequelae. If long-term support or destination therapy is anticipated, leaving the valve uncorrected may lead to premature pump failure (25).
Preferred therapies for long-term support include placing a new competent bioprosthetic valve, or complete ligation of the native valve cusps. As stated earlier, mechanical valves are avoided due to the possibility of stasis of the valve, subsequent thrombus formation and systemic embolization (25).
While temporary solutions include optimizing hemodynamics to mitigate severity of AI and potentially prevent aortic valve manipulation in the short term, there is a tendency for any degree of AI to worsen after LVAD implantation as pressures and volume within the LV are attenuated (26,27).
LVAD failure secondary to inadequate LV unloading and LV suction events
A benefit of ventricular assist devices includes the unloading of the LV to improve cardiac work as per Laplace’s law. Failure to adequately unload the LV may result in excessive wall stress on the LV. Clinically, signs of partial or inadequate LV unloading include increased pulsatility, visible in arterial tracing and/or non-invasive monitoring. In addition, as more work is transmitted to the native LV, signs and symptoms of LV overload such as increased filling pressures and pulmonary or systemic congestion, possible RV failure, and subsequent hemodynamic instability may occur.
Increased pulsatility with partial LV unloading on echocardiography translates to increased duration or more frequent opening of the aortic valve, usually with every ventricular beat. Using 2D- or 3D-echocardiography, increased size of the LV will be visualized, either by linear or volumetric measurements. For both aortic valve opening and LV dimensions, it is important to have baseline imaging to better identify changes in clinical conditions and for comparison during ramp testing post-implantation. Since inadequate unloading leads to an increased LV filling pressure and decreased flow through the LVAD, additional findings include an increase in left atrial size and possible new or worsening degree of mitral regurgitation. Further evaluation of mitral valve flow with Doppler evaluation may identify an increased mitral inflow peak E-wave diastolic velocity and decreased deceleration time of mitral E-wave velocity (28).
On the contrary, excessive LV unloading with disproportionate LVAD flows can lead to extreme LV decompression and subsequent hemodynamic instability. A suction event or “suck down” occurs when the walls of the LV collapse on itself following LVAD implantation (Figure 15). The myocardium obstructs normal blood flow into the inflow cannula as a result of either excessive LVAD device programmed speed or an under-filled LV from inadequate preload. Clinically, these events are manifested as minimal or no pulsatility, hypotension, and/or possible ventricular arrhythmias. On the device module low flow and increased Pulse Index (PI) alarms may be seen (29).
During these suction events echocardiographic findings will often exhibit a dramatic reduction in LV cavity dimensions; however, this singular finding does clarify the cause. Findings of RV failure on TEE would also support LV under-filling. While RV failure is typically associated with a deviation of the ventricular septum toward the LV, this may also be caused by an increased LVAD speed pulling the septum toward the device. Therefore, evaluation of overall RV function (tricuspid annular motion, RV free wall, and septum) is crucial in this setting. Decreasing the device speed or increasing preload will both increase LV size and potentially mitigate this effect. Finally, it is important to evaluate inflow cannula position, as malposition may be a consequence or cause of ongoing suction events. Inflow cannula malposition significant enough to cause repeated and significant suction events, usually warrants surgical intervention (6).
RV dysfunction
Maintaining proper RV function is vital after placing an LVAD to ensure adequate preload to the new device. Insufficient preload to the LV from the RV may produce a low output state, or even worse, device failure; therefore, proper assessment of RV function is paramount and is performed in a similar fashion to the assessment made pre-implant. Signs of a failing RV on TEE include a dilated RV, decreasing TAPSE (normal >16 mm), new onset TR, and decreased free wall motion. All these signs should be correlated with the pre-implant assessment. One study recently demonstrated a TAPSE of less than 12.5 mm as predictive of the need for an RVAD (84% sensitive, 75% specific) (30). Additionally, measuring an RV fractional area change (RVFAC) can help delineate RV dysfunction (6). An RVFAC of 40% or greater is normal, while most patients needing an LVAD fall in the 20–30% range. An RVFAC of less than 20% may indicate future RV failure (29). Once the chest is closed, cardiac tamponade may also cause RV compression leading to RV failure. Along with these findings, a TEE assessment will show an under filled, decompressed LV and LVAD cannula inflow obstruction from LV ‘suckdown’.
If the LVAD is working where return to the LV is insufficient for outflow output, the interventricular septum may bow towards the inflow cannula (LV). This geometric reshaping of the RV can again lead to poor RV function and decreased preload. If persistent RV dysfunction is present, it may be necessary to place a RV assist device, even as a temporary measure to allow time for the RV to acclimate to the new LVAD.
Intracardiac thrombus
As discussed earlier, unrecognized thrombus can lead to catastrophic device failure as a consequence of entrainment into the LVAD device as flows commence. Chen et al. were able to demonstrate the utility of TEE for the diagnosis of LV apical thrombus, demonstrating its ability to find thrombus (31). On the other hand, TTE has been shown to be less sensitive to detecting a left atrial thrombus which consequently may be missed on preoperative workup (32). Nevertheless, if suspicion for thrombus remains high and no thrombus is visualized, consideration should be given to use of a microbubble contrast agent to help delineate blood flow patterns within the ventricle (33).
Swift diagnosis of a thrombus is imperative in the post-implant phase. In addition to echocardiographic findings, it can be diagnosed by looking for signs of hemolysis; including LDH and bilirubin, and increased LVAD pump power requirements with a decline in pump flows. Signs and symptoms of congestive heart failure (CHF) may also be present. Reassessment with TEE may find additional clot that has not yet propagated to the pump. Additionally, the use of cardiac computed tomography or fluoroscopy post-surgery may aid in the diagnosis of thrombus formation undetected by TEE intraoperatively.
LVAD-related mitral regurgitation
Mitral regurgitation following LVAD placement is usually improved secondary to an overall increase in cardiac output, due specifically to the continuous flow generated by the device. Residual mitral regurgitation following LVAD placement can be functional in nature or can be secondary to increased LV filling pressures, indicating inadequate LV unloading or ongoing heart failure. Unique or primary to LVAD placement is the possible interference of the inflow cannula with the subvalvular mitral valve apparatus. This should be identified in the operating room since it often requires surgical intervention. When evaluating for causes of mitral regurgitation, color-flow Doppler should reveal low-velocity, laminar flow without signs of obstruction or turbulence (indicated by high velocities). For a continuous flow device, any turbulent color-flow Doppler signal or variability in velocities may represent a mechanical obstruction indicating that the septum, muscular trabeculations, and subvalvular mitral apparatus should be evaluated further (6).
Pericardial effusion
Similar to managements of other patients following cardiac surgery, hypotension warrants the investigation of a pericardial effusion and resulting cardiac tamponade. It is, however, important to remember that pericardial effusions may be seen in a normotensive or hemodynamically stable patient following cardiac surgery and LVAD placement. A pericardial effusion sizable enough to limit ventricular filling in the diastolic phase is cardiac tamponade and is a clinical diagnosis. Similar to patients without an LVAD, a pericardial effusion may be visible using both TTE and TEE. Postoperatively, TEE may be favorable due to an under appreciation of posterior fluid collections using TTE due to limited imaging windows, especially in the presence of mediastinal and pleural tube thoracostomy drains. Since LVADs generate a majority of the cardiac output, flow across the mitral valve and tricuspid valves are significantly influenced by the continuous flow. Compared to patients without an LVAD, paradoxical flow across the tricuspid and mitral valves, as classically seen with cardiac tamponade, may not be present and is unreliable (6). In addition to clinical suspicion, a fluid collection and signs of RV compression may be the only findings on echocardiography. The presence of RV compression does contribute to the diagnosis of tamponade in the differential of other causes of hypotension in the patient with an LVAD (29). Clinical routine practice, therefore is to investigate for the development of pericardial effusions after chest closure, and before exiting the operating room with echocardiographic images scanning the lateral walls of the four chambers of the heart.
Conclusions
Intraoperative TEE for placement of continuous-flow left ventricular assist devices (CF-LVADs) is necessary and important for the long-term success of this patient population. From the initial intraoperative assessment to arrival in the intensive care unit, TEE plays a vital role in providing essential information to optimize management of a patient with a newly inserted device and have a positive impact on patient outcomes. TEE can provide a wealth of information on the changes incurred on the anatomy of the heart such as the ventricles, valves, and great vessels, and on hemodynamics altered by the new physiology created by the device. However, the echocardiographer must have a detailed understanding of LVADs and their alteration on the patient’s physiology in addition to astute experience in TEE for proper evaluation.
The number of patients presenting for LVAD support for long- or short-term support will continue to increase due to population increases and technological advances allowing for more sophisticated and less invasive devices. The role of TEE will continue to be a vital instrument for appraising the continual hemodynamic changes and examining the effectiveness of the device while helping diagnose issues that need resolution. It is the onus of the TEE examiner to have an in-depth knowledge of not only standard assessments but also a complete understanding of issues that may arise during LVAD implantation (6).
We have detailed the echocardiographic assessment particular to the patient presenting for LVAD implantation. This assessment is in addition to the recommended comprehensive TEE exam for any patient presenting for an intracardiac procedure (5). The normal echocardiographic findings after LVAD implantation are outlined with a discussion regarding unexpected findings or complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Stevenson LW, Miller LW, Desvigne-Nickens P, et al. Left ventricular assist device as destination for patients undergoing intravenous inotropic therapy: a subset analysis from REMATCH (Randomized Evaluation of Mechanical Assistance in Treatment of Chronic Heart Failure). Circulation 2004;110:975-81. [PubMed]
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001;345:1435-43. [PubMed]
- Todaro MC, Khandheria BK, Paterick TE, et al. The practical role of echocardiography in selection, implantation, and management of patients requiring LVAD therapy. Curr Cardiol Rep 2014;16:468. [PubMed]
- Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant 2015;34:1123-30. [PubMed]
- Hahn RT, Abraham T, Adams MS, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013;26:921-64. [PubMed]
- Stainback RF, Estep JD, Agler DA, et al. Echocardiography in the Management of Patients with Left Ventricular Assist Devices: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2015;28:853-909. [PubMed]
- Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009;53:e1-e90. [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [PubMed]
- Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2010;105:1030-5. [PubMed]
- Lee S, Kamdar F, Madlon-Kay R, et al. Effects of the HeartMate II continuous-flow left ventricular assist device on right ventricular function. J Heart Lung Transplant 2010;29:209-15. [PubMed]
- Estep JD, Stainback RF, Little SH, et al. The role of echocardiography and other imaging modalities in patients with left ventricular assist devices. JACC Cardiovasc Imaging 2010;3:1049-64. [PubMed]
- Matthews JC, Koelling TM, Pagani FD, et al. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 2008;51:2163-72. [PubMed]
- Ochiai Y, McCarthy PM, Smedira NG, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation 2002;106:I198-202. [PubMed]
- Kato TS, Jiang J, Schulze PC, et al. Serial echocardiography using tissue Doppler and speckle tracking imaging to monitor right ventricular failure before and after left ventricular assist device surgery. JACC Heart Fail 2013;1:216-22. [PubMed]
- Cameli M, Righini FM, Lisi M, et al. Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. Am J Cardiol 2013;112:1778-84. [PubMed]
- Kato TS, Farr M, Schulze PC, et al. Usefulness of two-dimensional echocardiographic parameters of the left side of the heart to predict right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2012;109:246-51. [PubMed]
- Grant AD, Smedira NG, Starling RC, et al. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol 2012;60:521-8. [PubMed]
- Piacentino V 3rd, Ganapathi AM, Stafford-Smith M, et al. Utility of concomitant tricuspid valve procedures for patients undergoing implantation of a continuous-flow left ventricular device. J Thorac Cardiovasc Surg 2012;144:1217-21. [PubMed]
- Cohn WE. New tools and techniques to facilitate off-pump left ventricular assist device implantation. Tex Heart Inst J 2010;37:559-61. [PubMed]
- Chumnanvej S, Wood MJ, MacGillivray TE, et al. Perioperative echocardiographic examination for ventricular assist device implantation. Anesth Analg 2007;105:583-601. [PubMed]
- Essandoh M. Utility of Three-dimensional Echocardiography for Implantation of a Left Ventricular Assist Device. Anesthesiology 2015. [Epub ahead of print]. [PubMed]
- May-Newman KD, Hillen BK, Sironda CS, et al. Effect of LVAD outflow conduit insertion angle on flow through the native aorta. J Med Eng Technol 2004;28:105-9. [PubMed]
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157-87. [PubMed]
- Dranishnikov N, Stepanenko A, Potapov EV, et al. Simultaneous aortic valve replacement in left ventricular assist device recipients: single-center experience. Int J Artif Organs 2012;35:489-94. [PubMed]
- Rao V, Slater JP, Edwards NM, et al. Surgical management of valvular disease in patients requiring left ventricular assist device support. Ann Thorac Surg 2001;71:1448-53. [PubMed]
- Cowger J, Pagani FD, Haft JW, et al. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail 2010;3:668-74. [PubMed]
- Bryant AS, Holman WL, Nanda NC, et al. Native aortic valve insufficiency in patients with left ventricular assist devices. Ann Thorac Surg 2006;81:e6-8. [PubMed]
- Estep JD, Chang SM, Bhimaraj A, et al. Imaging for ventricular function and myocardial recovery on nonpulsatile ventricular assist devices. Circulation 2012;125:2265-77. [PubMed]
- Pratt AK, Shah NS, Boyce SW. Left ventricular assist device management in the ICU. Crit Care Med 2014;42:158-68. [PubMed]
- Patil NP, Mohite PN, Sabashnikov A, et al. Preoperative predictors and outcomes of right ventricular assist device implantation after continuous-flow left ventricular assist device implantation. J Thorac Cardiovasc Surg 2015;150:1651-8. [PubMed]
- Chen C, Koschyk D, Hamm C, et al. Usefulness of transesophageal echocardiography in identifying small left ventricular apical thrombus. J Am Coll Cardiol 1993;21:208-15. [PubMed]
- Scalia GM, McCarthy PM, Savage RM, et al. Clinical utility of echocardiography in the management of implantable ventricular assist devices. J Am Soc Echocardiogr 2000;13:754-63. [PubMed]
- Abdelmoneim SS, Pellikka PA, Mulvagh SL. Contrast echocardiography for assessment of left ventricular thrombi. J Ultrasound Med 2014;33:1337-44. [PubMed]