|
Clinical Trials Notes
Feasibility Of Administering Adjuvant Chemotherapy Of Pemetrexed Followed By Pemetrexed/oxaliplatin Immediately Post -VATS In Patients With Completely Resected NSCLC
Jianxing He, Wenlong Shao, Shuben Li, Manyin Chen, Hanzhang Chen, Jun Liu, Wei Wang, Yuan Qiu, Daoyuan Wang
From Department of Cardiothoracic Surgery, the First A ffiliated Hospital of Guangzhou Medical College; Guangzhou Research Institute of Respiratory Disease & China State Key Laboratory of Respiratory Disease, Guangzhou, PR China
Corresponding to: Prof. Dr. Jianxing He, MD, PhD, FACS. Department of Cardiothoracic
Surgery, The First Affiliated Hospital of Guangzhou Medical College; Guangzhou Research
Institute of Respiratory Disease & China State Key Laboratory of Respiratory Disease. No.151,Yanjiang Rd,Guangzhou 510120,
PR China.Tel:+86-20-83337792,Fax:+86-20-83350363, Email: hejx@vip.163.com
|
|
Abstract
Non-small cell lung cancer (NSCLC) accounts for the largest number of cancer deaths annually, worldwide. It seems reasonable to test a
less toxic regimen also in early stages after complete (R0) resection of the tumor, where reduced toxicities might improve the feasibility of
drug delivery, compliance and the convenience of treatment for the patient and hence perhaps improve survival. The main purpose of this
phase II trial is to evaluate the clinical feasibility-in terms of patients without dose limiting toxicities or premature treatment withdrawal or
death-of administering adjuvant chemotherapy of pemetrexed followed by pemetrexed/oxaliplatin immediately post-VATS (video-assisted
thoracic surgery) in patients with completely resected NSCLC.
Key words
non-small cell lung cancer; video-assisted thoracic surgery; adjuvant chemotherapy; pemetrexed; oxaliplatin
J Thorac Dis 2009;1:55-62. DOI: 10.3978/j.issn.2072-1439.2009.12.01.002
|
|
Introduction
Non-small cell lung cancer (NSCLC) accounts for the largest
number of cancer deaths annually, worldwide ( 1). Of these,
about 30% are early stage patients (stage I and II). For this group
of patients, radical surgery with mediastinal lymph node dissection has been the mainstay of therapy with a reasonable curative
option. However, 5-year survival rates for patients with pathologically staged IA-IIB disease are ranging from 67% to 39% ( 2).
Following surgery, distant recurrence is the most common form
of relapse and eventual cause of death. Assuming that these recurrences are due to occult micrometastases at the time of
surgery, trials on adjuvant systemic therapy have been performed
in an attempt to reduce the risk of recurrence and to improve survival.
In some of the recently published trials a clear benefit of adjuvant chemotherapy in early stage NSCLC could not be achieved
( 3-5). In marked contrast to these studies, three recent, big randomized trials on early stage NSCLC patients with modern platin-based
two-drug chemotherapy-regimens revealed a significant advantage
for overall or relapse free survival for chemotherapeutically treated
patients ( 6-8). The majority of patients in the adjuvant treatment
setting received a combination of cisplatin and vinorelbine. A
pooled analysis of five big randomized studies demonstrated that
adjuvant cisplatin-based chemotherapy significantly improves survival in patients with NSCLC (overall HR of death 0.89, P=0.005)
corresponding to a 5-year absolute benefit of 5.4% from
chemotherapy ( 9). However, toxicity and inadequate dose delivery
have been critical issues in all trials performed so far. Grade 3/4
toxicities are observed up to 73% with rates of neutropenic fever
up to 7%. Up to 77% of the patients had at least one dose reduction
or omission and 55% required one dose delay or more, most related to neutropenia ( 7, 10).
There are few data in the literature about how soon after surgery
a patient begins adjuvant chemotherapy, although most trials seem
to start after a post-surgical interval of 4-6 weeks. A recent study reported that 26 patients, who underwent thoracoscopic (video-assisted thoracic surgery, VATS) lobectomy, receiving chemotherapy, 73% completed a full course on schedule and 85% received
all intended cycles ( 11). In another study, complete resection was
performed by thoracotomy in 43 patients and by thoracoscopy in
57 patients, compared with thoracotomy, patients undergoing
thoraco scopic lobectomy had significantly fewer delayed (18%
versus 58%, P < 0.001) and reduced (26% versus 49%, P = 0.02)
chemotherapy doses. A higher percentage of patients undergoing
thoracoscopic resection received 75% or more of their planned adjuvant regimen without delayed or reduced doses (61% versus 40% , P = 0.03). There were no significant differences in time to
initiation of chemotherapy or toxicity ( 12). In comparison, the Cancer and Leukemia Group B trial 9633 reported that 57% of patients
received full-dose chemotherapy ( 13) and the Intergroup JBR.10
trial reported that 55% of patients had at least 1 dose delay ( 7). Approximately 34% of patients in the Adjuvant Lung Project Italy series chemotherapy wing received all scheduled doses without adjustment or delay; 69% completed their treatments with or without
adjustments or delay ( 4). It is conceivable that patients who undergo VATS may have a quicker recovery and in general more
strength to tolerate chemotherapy. There are theoretic survival benefits to starting chemotherapy immediately after surgery because
the body’s tumor burden should be lowest, and tumor growth
fastest, at this time. Thus, chemotherapy administered immediately
post-surgery would be most effective, assuming that wound healing
is adequate ( 11).
Pemetrexed, a multi-target folate antimetabolite, shows clear activity in non-small cell lung cancer. In a phase III study for patients
with previously treated advanced non-small cell lung cancer, the
efficacy of single-agent pemetrexed, as determined by overall survival, was similar to that of docetaxel ( 14). The combination of oxaliplatin and pemetrexed has been of particular interest because it
has demonstrated both good efficacy and a tolerable side effect
profile. Oxaliplatin is a diaminocyclohexane-containing platinum
compound that inhibits DNA replication and transcription by forming DNA adducts. Its mechanism of action is similar to that of the
classic platinum drugs, but molecular pharmacology studies suggest that oxaliplatin represents a distinct family of platinum compounds. It has a different cytotoxicity profile from cisplatin and can
be safely given in the outpatient setting without hydration therapy
( 15). Moreover, oxaliplatin appears to interact synergistically with
pemetrexed ( 16). Phase I studies evaluated pemetrexed plus oxaliplatin in patients with solid tumors, and showed the regimen was
efficacious and well tolerated ( 17). The combination of oxaliplatin
and pemetrexed was compared with carboplatin and pemetrexed as
first-line therapy for advanced NSCLC in a randomized phase II
study. Response rates were 26.8 and 31.6%, respectively, and not
statistically different. However, toxicity in the oxaliplatin/pemetrexed arm was quite low, this doublet can be delivered easily and
is well tolerated. Furthermore, it results in a 7.3% rate of grade 3/4
neutropenia only and the incidence of febrile neutropenia was
2.4%. Dose reductions occur only in 2.6% cycles. Patients received
95.3% and 100% of the planned weekly mean doses of pemetrexed
and oxaliplatin, respectively ( 18).
Therefore, it seems reasonable to test a less toxic regimen also
in early stages after complete (R0) resection of the tumor, where
reduced toxicities might improve the feasibility of drug delivery,
compliance and the convenience of treatment for the patient and
hence perhaps improve survival. The main purpose of this phase II
trial is to evaluate the clinical feasibility-in terms of patients without dose limiting toxicities or premature treatment withdrawal or
death-of administering adjuvant chemotherapy of pemetrexed followed by pemetrexed/oxaliplatin immediately post-VATS in patients with completely resected NSCLC.
|
|
Objectives and Outcome Measures
2.1 Primary Outcome Measures:
This study is a prospective phase II study determining the clinical feasibility in terms of toxicity of 4 cycles of adjuvant
chemotherapy with pemetrexed followed by pemetrexed/oxaliplatin in patients with completely resected non-squamous NSCLC
(stage IB, IIA, IIB and IIIA) by VATS, after a postersurgical interval of 2-4 weeks, to estimate whether patients who undergo VATS
may have a quicker recovery and in general more strength to tolerate chemotherapy.
The primary objective is to determine the clinical feasibility rate
(CFR) of 4 cycles of adjuvant chemotherapy with pemetrexed followed by pemetrexed/oxaliplatin in patients with NSCLC stage IB,
IIA, IIB and IIIA after a postersurgical interval of 2-4 weeks. Treatment is considered to have clinical feasibility if dose limiting toxicity (DLT) will not be observed, and no non-acceptance by the patient leading to premature withdrawal, and no death due to cancer
or cancer therapy will occur.
DLTs are defined as:
- Grade 4 neutropenia more than 7 days
- Grade 4 thrombocytopenia more than 7 days
- Grade 3/4 neutropenia with fever (i.e. > 38.5° C on at least 2
occasions in 24 hours time) and/or infection (i.e. documented by
either culture or imaging method)
- Any grade thrombocytopenia with bleeding
- Grade 3/4 non-hematological toxicity possibly or probably re-
lated to the chemotherapy (except for nausea/vomiting/hair loss)
2.2 Secondary Outcome Measures:
Secondary objectives are to determine the time to treatment failure,
the relapse free survival, the overall survival, the distant
metastases free survival, local relapse free survival, the localization
of relapse.
|
|
Statistical Consideration
This phase II trial design is based on the following assumptions:
the experimental therapy arm would be rated as unacceptable, if
the actual feasibility rate (= 1–withdrawal/DLT rate) was 65 % or
lower. On the other hand, the therapy would be considered to be a
promising candidate for further development, if the true feasibility
rate amounted to 80% or more. Probability to accept the experimental therapy as well tolerable, in spite of a true feasibility rate of
< 65% (i.e. withdrawal/DLT rate > 35%): 5% (type I error). Probability to reject the experimental therapy as not sufficiently feasible
(< 65%), although the true feasibility rate is promising (> 80%):20% (type II error, corresponding to a power of 80%). According
to these parameters, and using the variant out of the class of optimal two-stage designs by Simon that leads to the lowest maximum
number of patients required (minimax approach), n = 18 patients
evaluable for feasibility have to be recruited in the first stage ( 19).
The combination will be rejected, if three or more of these patients
fulfil the criterion of non-feasibility. In the second step, further patients will be recruited up to a total number of 67 cases. Allowing
for a follow-up loss rate of 10%, the total sample size was 75 patients.
The final conclusion of the trial will depend on the definite feasibility rate (and its confidence interval), the achieved level of drug
delivery as well as the complete information on type, frequency
and severity of toxicities. Event related data like relapse-free or
overall survival will be estimated by the product limit method by
KAPLAN-MEIER CURVE and compared using the log-rank test.
The methods mentioned above are likewise suitable for the univariate evaluation of prognostic factors. Multivariate analyses may be
performed by suitable regression models (COX proportional hazard
regression model, logistic regression).
|
|
Patient Selection
4.1 Inclusion Criteria:
Patients are eligible to be included in the study only if they meet
all of the following criteria:
● Patients with completely resected stage IB (>4 cm), II, or IIIA
non-squamous NSCLC by VATS. Patient must be enrolled and begin therapy within 4 weeks from the date of complete surgical resection.
● Fresh tissue must be available for genomics expression profiling.
● ECOG performance status of 0 or 1.
● No prior chemotherapy, radiation therapy, or biologic/targeted therapy within the last 5 years. Prior therapy with low dose
methotrexate or similar medications is allowed if therapy used to
treat non-malignant conditions.
● Age ≥ 18 years.
● No previous or concomitant malignancy in the past 5 years
other than curatively-treated carcinoma in situ of the cervix, or
basal cell or squamous cell carcinoma of the skin.
● No other serious medical or psychiatric illness.
● Signed informed consent.
● Required laboratory data within one week of enrollment:
■ ANC or AGC >= 1500 per uL;
■ Platelets >= 100,000 per uL;
■ Total bilirubin <= 1.5 mg/dL;
■ Creatinine < 2 mg/dL; creatinine clearance >= 45 mL/min;
■ SGOT/SGPT <= 1.5*ULN.
● Females of child-bearing potential (not surgically sterilized
and between menarche and 1 year post menopause) must test negative for pregnancy within 7 days prior to or at the time of enrollment based on a serum pregnancy test. Both sexually active males
and females of reproductive potential must agree to use a reliable
method of birth control, as determined by the patient and their
health care team, during the study and for 3 months following the
last dose of study drug.
4.2 Exclusion Criteria:
Patients will be excluded from the study if they meet any of the
following criteria:
● Treatment within the last 30 days with a drug that has not received regulatory approval for any indication at the time of study
entry.
● Concurrent administration of any other anti-tumor therapy.
● Inability to comply with protocol or study procedures.
● Active infection requiring IV antibiotics, antifungal or antiviral agents, that in the opinion of the investigator would compromise the patient's ability to tolerate therapy.
● Major surgery (other than definitive lung cancer surgery)
within two weeks of study or other serious concomitant systemic
disorders that, in the opinion of the investigator, would compromise the safety of the patient or compromise the patient's ability to
complete the study.
● Myocardial infarction having occurred less than 6 months before inclusion, any known uncontrolled arrhythmia, symptomatic
angina pectoris, active ischemia, or cardiac failure not controlled
by medications.
● Contraindication to corticosteroids.
● Inability or unwillingness to take folic acid or vitamin B12
supplementation.
● Unwillingness to stop taking herbal supplements while on
study.
● Presence of clinically significant third-space fluid collections
(for example, ascites or pleural effusions) that cannot be controlled
by drainage or other procedures prior to study entry and throughout
study enrollment as the distribution of pemetrexed in this fluid
space is not fully understood.
● Inability to discontinue administration of aspirin at a dose >
1300 mg/day or other long acting, non-steroidal anti-inflammatory
agents for 2 days before, the day of, and 2 days after the dose of
pemetrexed (5 days prior for long-acting agents such as piroxicam). Moderate dose ibuprofen may be continued.
● Female patients that are pregnant or breast-feeding.
|
|
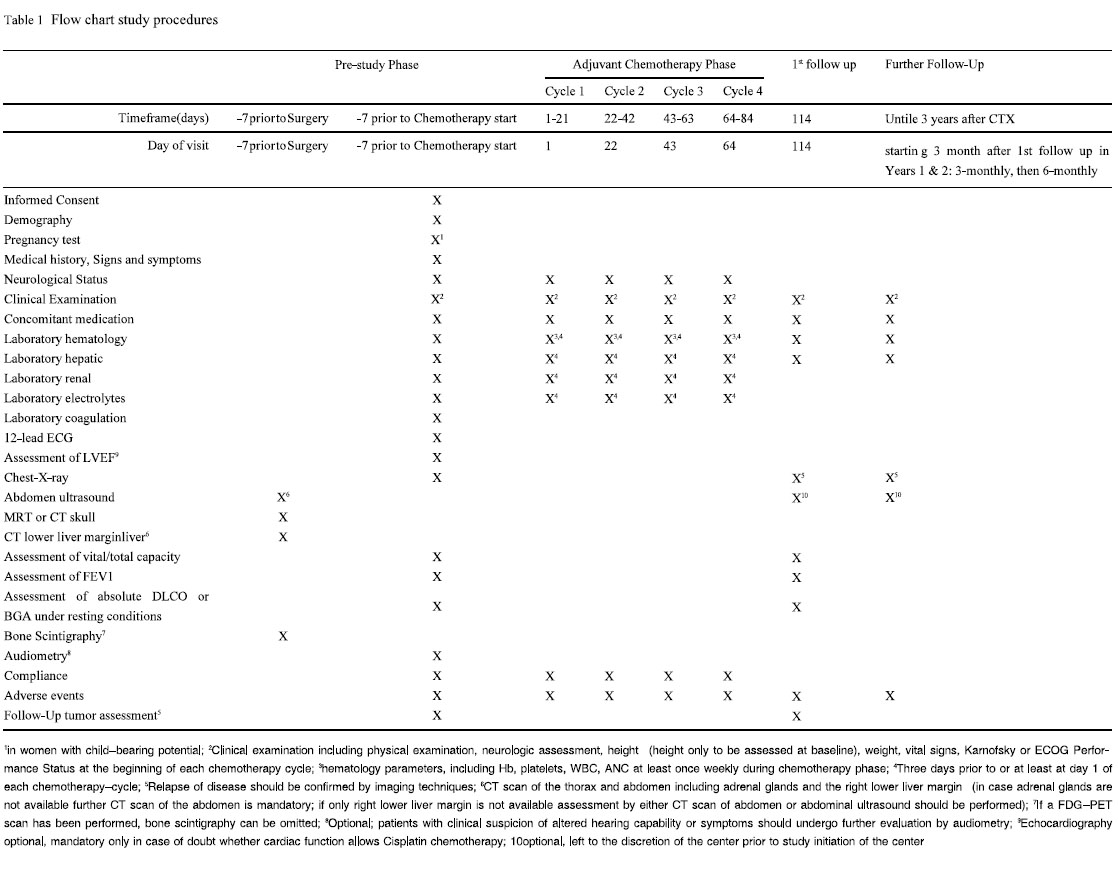
Pretreatment Evaluation
The following assessments will be performed before, during and
after the study ( Table 1). Optimal preoperative staging should have
consisted of the following procedures and should have been assured at least in a time interval starting 7 days prior to surgery: CT
scan of the thorax and abdomen including adrenal glands and the
right lower liver margin (in case adrenal glands are not available
further CT scan of the abdomen is mandatory; if only right lower
liver margin is not available assessment by either CT scan of abdomen or abdominal ultrasound should be performed), MRT
(preferably) or CT scan of the skull and bone scintigraphy (if a
FDG-PET scan has been performed bone scintigraphy can be omitted).
Not more than 7 days prior to the start of chemotherapy, for all
patients the following baseline parameters will be assessed: patient
demography, clinical examination including physical examination,
height, weight, vital signs, ECOG Performance Status, assessment
of the neurological status, existing signs and symptoms, medical
history (including concurrent illnesses) and specific details on the
diagnosis of non-small cell lung cancer (NSCLC), previous anti-cancer therapy and their outcome, concomitant medication, laboratory
assessment including hematological parameters
(hemoglobin, WBC, ANC, platelets), electrolytes (Na, K, Ca), hepatic parameters (total bilirubin, ASAT, ALAT, AP, gGT, LDH), renal parameters (creatinine, calculated creatinine clearance, Urea,
uric acid), coagulation parameters (Quick, PTT, Fibrinogen), and
pregnancy test for women with childbearing potential. Furthermore
chest-X-ray, electrocardiography and optional echocardiograph
will be performed.
Pulmonary function will be assessed by FEV1, vital capacity
and total capacity and by either absolute DLCO or capillary/arterial
BGA in resting condition with absolute DLCO > 40 % or pO2 > 60
mmHg in resting condition. Patients with clinical suspicion of altered hearing capability or symptoms, e.g. tinnitus, should undergo
further evaluation by audiometry. If preoperative staging had not
comprised all mandatory procedures it should be completed as outlined above before registration.
|
|
Registration Procedures
The current phase II trial is a single institution study. All patients should be seen by principle investigator (Dr Jianxing He,
MD, PhD, FACS), and should be registered in the Department
of Cardiothoracic Surgery, Guangzhou Research Institute of Respiratory Disease, China State Key Laboratory of Respiratory Disease, the First Affiliated Hospital of Guangzhou Medical College.
Every patient must provide a written informed consent to the trial procedures. The patient must be informed verbally and by the
provided patient information by the investigator before informed
consent is obtained.
|
|
Chemotherapy
In the first cycle, patients received Pemetrexed 500 mg/m2 (i.v.
infusion over 10 minutes) on day 1 of a 21-day cycle. From 2nd cycle, patients received pemetrexed 500 mg/m2 (i.v. infusion over 10
minutes) then oxaliplatin 120 mg/m2 (i.v. infusion over 120 minutes) on day 1 of a 21-day cycle. Study drug administration is to
begin on d14 to d28 after R0 resection of the tumor. A total of
three cycles is intended for patients with stage IB NSCLC, and four
cycles for II-IIIA NSCLC, respectively.
Folic acid (350-1000 μg) must be given daily beginning approximately 5-7 days prior to first dose of pemetrexed and continuing
daily until 3 weeks after the last dose of study therapy. Vitamin
B12 (1000 μg) will be administered as an intramuscular injection
approximately 1 to 2 weeks prior to first dose of pemetrexed and
repeated approximately every 9 weeks until 3 weeks after the last
dose of study therapy. Dexamethasone (4 mg of oral or equivalent)
given twice daily should be taken on the day before, the day of,
and the day after each dose of pemetrexed, for rash prophylaxis unless medically contraindicated.
|
|
Toxicity management
Toxicities are classified by grade, type, duration, onset, and relationship to study treatment according to CTCAE version 3.0. After application of chemotherapy blood count should be performed
at least once weekly. In case of leukocytopenia or neutrocytopenia
CTC grade 4 (leukocytes < 1.0× 109/L), antibiotic prophylaxis according to local habits is recommended. Prophylaxis should be
used similarly in both treatment arms. Routine use of colony-stimulating factor (CSF) is not permitted during this study. ASCO guidelines for use of CSF should be followed ( 20). Granulocyte colony
stimulating factor must have been discontinued at least 24 hours
prior to the start of the next chemotherapy infusion. In case of
thrombocytopenia CTC grade 4 the patient will be discontinued
from the study. In the event of CTC Grade 3 or 4 diarrhea, the following supportive measures are allowed: hydration, octreotide, and
antidiarrheals. If diarrhea is severe (requiring intravenous rehydration) and/or associated with fever or severe neutropenia (Grade 3
or 4), broad-spectrum antibiotics must be prescribed. Patients with
severe diarrhea or any diarrhea associated with severe nausea or
vomiting must be hospitalized for intravenous hydration and correction of electrolyte imbalances. Patients with CTC grade 3 or 4
diarrhea will be discontinued from the study. Professional supervised oral care protocols that include patient education in an attempt to reduce the severity of mucositis from chemotherapy is
highly advised to all patients. In case of mucositis related pain,
symptomatic therapy according to WHO guidelines should be performed. Based on their proposed mechanisms, antimicrobial agents, such as combined polymyxin E, tobramycin, and amphotericin or single-agent iseganan, appear to have no associated
mechanistic rationale for the prevention of mucositis and probably
could provide benefit only for patients with late-stage ulcerative
mucositis, in which bacterial superinfection occurs. The use of amphotericin suspension at clinical signs of mucosal soor and at mucositis Grad 3/4 is recommended. For patients receiving pemetrexed leucovorin should be considered. Patients with CTC grade 3
or 4 mucositis will be discontinued from the study.
|
|
Dose adjustments, safety and discontinuation of treatment
The dose reductions are limited to a preset pattern, and eliminate the need to make any calculations or resolve conflicting recommendations
if two or more toxicities occur within the same cycle. It also ensures that therapeutic chemotherapy doses will be administered
in all treatment cycles. Patients should be instructed to
report any toxicity that occurs during drug administration of each
treatment course and in the period between cycles. Treatment will
be modified in case of hematological and/or non-hematological
toxicities. All dose adjustments will be made according to the system showing the greatest degree of toxicities. Toxicities will be
graded according to the NCI Common Toxicity criteria (CTCAE
version 3.0). No dose re-escalation will be performed after dose reduction. If the study treatment cannot be administered after an
additional 2 weeks delay because of any toxicity, it should be definitively discontinued.
On day 1 of each cycle, the following criteria have to be met for
the administration of pemetrexed or oxaliplatin/pemetrexed: ANC
= 1,500/μl, Platelets = 100,000/μl, Serum creatinine < 1.5 mg/dl
and calculated creatinine clearance = 60 ml/min, no other grade =
2 toxicity (except for clinically non-relevant AEs such as alopecia,
altered taste, nausea, vomiting). If these criteria are not met, drug
administration has to be delayed up to 1 week to allow for recovery. If a delay of more than 14 days due to toxicity is necessary, the
patient is to be discontinued from the study.
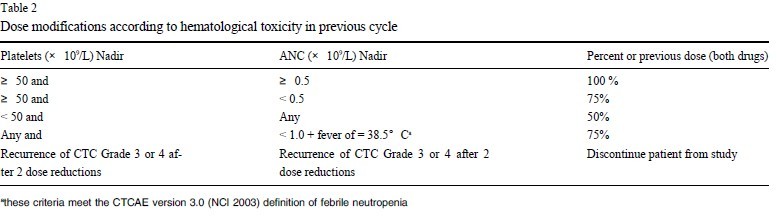
Dose adjustments according to hematological toxicity at the
start of a subsequent course of the therapy will be based on platelet
and neutrophil nadir counts from the preceding cycle of therapy.
No dose modifications will be made for anemia. ANC must be =
1,500/μl and platelets =100,000/μl as outlined above prior to the
start of any cycle. Treatment may be delayed to allow sufficient
time for recovery. Upon recovery patients should be re-treated using the guidelines as outlined in table 2.
Any patient experiencing Grade 3/4 non-hematological toxicity
(except for nausea/vomiting/hair loss) associated with therapy will
be discontinued from the study. In case of neurological and/or
hearing CTC grade 2 toxicity, administration should be delayed
and reassessed one week later. If toxicity has resolved at least to
grade 1 therapy should be continued with 50% dose reduction for
oxaliplatin for further administrations. In case of decrease of calculated creatinine clearance, despite adequate hydration, administration has to be delayed and reassessed one week later and if the value of creatinine clearance remains < 60 ml/min, patient has to be
discontinued.
For patients who develop clinically significant pleural or peritoneal effusions (on the basis of symptoms or clinical examination)
during therapy, consideration should be given to drain the effusion
prior to therapy. Though, if, in the investigator's opinion, the effusion represents relapse and/or progression of disease, the patient
should be discontinued from study therapy. However, disease relapse has to be confirmed by adequate imaging methods. Patients
with sero (pneumo)-thorax after hemi-pneumonectomy or lobectomy will not be excluded. Those patients must be monitored for toxicity closely.
Dose reductions for hepatic dysfunction will be based on bilirubine and/or transaminase values. For bilirubin values 1.5-3 × UNL
and ASAT/ALAT < 5 × UNL, vinorelbine has to be reduced to
50%. For Grade 3/4 hepatic toxicity the patient has to be discontinued from study.
|
|
Premature withdrawal
Patients have the right to withdraw from the study at any time
for any reason. The investigator also has the right to withdraw patients from the study in the event of intercurrent illness, adverse
events, protocol violations, administrative reasons or other reasons.
Discontinuation of a patient should be based upon one of the objective toxicity criteria. The following reasons can lead to premature withdrawal from therapy of a patient: major protocol violation,
Non-compliance of a patient with protocol procedures, unacceptable toxicity according to the protocol, unacceptable toxicity as
perceived by the patient (refusal to continue), withdrawal of consent by the patient, lost to follow-up, pregnancy, medical decision
by the investigator, appropriate evaluation demonstrates recurrence
of disease. The reason for withdrawal of a patient needs to be documented. Patients who have been withdrawn from therapy will
have to be further documented for follow-up.
|
|
VATS
VATS will be performed prior to chemotherapy. The surgical principles follow the guidelines of oncologic surgery. The tumor
and all intrapulmonary lymphatic drainage must be removed completely, employing lobectomy or pneumonectomy or complex resections, if necessary.
En bloc resection of closely adjacent or invaded structures is preferential to a discontinuous resection. Resection margins should be assessed
by frozen section analysis whenever possible; this includes bronchial, vascular and other margins with close proximity to the tumor if necessary
to obtain radical resection. Re-excision is preferred whenever possible, if positive resection margins are encountered. To achieve uncomplicated
bronchus- and trachealhealing stump or anastomoses (sleeve resection) can be covered with viable tissue (pedicled pleural, pericardial, intercostals
muscle flap or others). Considering the criteria of functional operability, the aim is to obtain R0-resection. All accessible hilar and mediastinal
lymph nodes should be removed for pathologic evaluation, using the technique of mediastinal lymph node dissection.
The systematic nodal dissection (SND) will be performed in a
standardized manner same as the previous report ( 21, 22). All the
surrounding fat containing the lymph tissue is isolated and removed systematically. Lymph nodes are to be identified and properly labelled by the surgeon. Tumor infiltration of lymph nodes
may not be apparent and is recommended to be diligently sought.
Microscopic assessment is required to accurately determine the
N-status and should be performed as described in the previous report ( 23). Complete mediastinal lymph node dissection is defined
as when all tissue containing lymph nodes is removed at all levels
accessible within the operation. Reflecting lymph node involvement, R0-resection is defined by the absence of tumor infiltration
in the most distal lymph node level removed according to the previous report ( 24). If possible, the most distant level should be the
upper-paratracheal lymph nodes (Level 2). The natural course of a
developing sero (pneumo)-thorax in case of pneumonectomy or
lobectomy is not regarded as a exclusion criteria. However, patients must be followed up closely for developing toxicities.
|
|
Radiotherapy
Radiotherapy is not planned.
|
|
Maintenance Therapy and Follow Up Period
No maintenance therapy is allowed. Follow-up visits are
planned starting at 30 days after the end of the last chemotherapy
cycle and afterwards in 3 monthly intervals for the first 2 years. In
the 3rd year patients will be followed up in 6 monthly intervals
( Table 1). The follow up visits comprise clinical examination including physical examination, neurologic assessment, weight, vital
signs, ECOG Performance Status, laboratory assessment including
hematological parameters (hemoglobin, WBC, ANC, platelets),
hepatic parameters (total bilirubin, ASAT, ALAT, AP, LDH), chest
X-ray, with further examinations in case of clinical symptoms, to
confirm relapse by imaging techniques, abdominal ultrasound, concomitant medication. Additional assessments in the 1st follow up at
30 days after the end of the last chemotherapy cycle comprise assessment of FEV, of vital and total capacity, capillary or arterial
BGA under resting conditions, absolute DLCO and adverse events.
Further examinations in case of clinical symptoms to confirm relapse by imaging techniques.
|
|
Trial duration
Individual participation is completed either three years after enrolment or death of the patient. Duration of the study is about 4 years.
|
|
Pathology
Pathology specimen (fresh tissue or paraffin block) is not needed for testing in the current study. However, efforts should be made
to retain fresh tissues for gene profiling in the future against the
treatment outcome for the potential identification of the gene signature that may predict the treatment outcome and patients'prognoses.
|
|
Data Collection
All finding on weekly or follow-up examination are to be entered to the patient data sheet specific for this study, and the
database collectively controlled by Dr Jianxing He, MD, FACS.
|
|
ClinicalTrials.gov Registration
|
|
References
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581-92.
[LinkOut]
- Mountain CF. Revisions in the International System for Staging Lung Cancer.Chest 1997;111:1710-7.
[LinkOut]
- Tada H, Tsuchiya R, Ichinose Y, Koike T, Nishizawa N, Nagai K, Kato H. A randomized trial comparing adjuvant chemotherapy versus surgery alone for completely resected pN2 non-small cell lung cancer (JCOG9304). Lung Cancer 2004;43:167-73.
[LinkOut]
- Scagliotti GV, Fossati R, Torri V, Crinò L, Giaccone G, Silvano G, et al; Adjuvant Lung Project Italy/European Organisation for Research Treatment of Cancer-Lung Cancer Cooperative Group Investigators. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst 2003;95:1453-61.
[LinkOut]
- Waller D, Peake MD, Stephens RJ, Gower NH, Milroy R, Parmar MK, et al.Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg 2004;26:173-82.
[LinkOut]
- Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J;International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60.
[LinkOut]
- Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, et al; National Cancer Institute of Canada Clinical Trials Group; National Cancer Institute of the United States Intergroup JBR. 10 Trial Investigators. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97.
[LinkOut]
- Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzá les-Larriba JL, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27.
[LinkOut]
- Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al; LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9.
[LinkOut]
- Pisters KMW, Le Chevalier T. Adjuvant Chemotherapy in completely resected non-small cell lung cancer. J Clin Oncol 2005 23:3270-8.
[LinkOut]
- Nicastri DG, Wisnivesky JP, Litle VR, Yun J, Chin C, Dembitzer FR, Swanson SJ. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg 2008;135:642-7.
[LinkOut]
- Petersen RP, Pham D, Burfeind WR, Hanish SI, Toloza EM, Harpole DH Jr, D'Amico TA. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9
[LinkOut]
- Strauss GM, Herndon JE 2nd, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51.
[LinkOut]
- Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97.
[LinkOut]
- Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol 1998;9:1053-71.
[LinkOut]
- Raymond E, Louvet C, Tournigand C, Coudray AM, Faivre S, De Gramont A, Gespach C. Pemetrexed disodium combined with oxaliplatin, SN38, or 5-fluorouracil, based on the quantitation of drug interactions in human HT29 colon cancer cells. Int J Oncol 2002;21:361-7.
[LinkOut]
- Misset JL, Gamelin E, Campone M, Delaloge S, Latz JE, Bozec L, Fumoleau P. Phase I and pharmacokinetic study of the multitargeted antifolate pemetrexed in combination with oxaliplatin in patients with advanced solid tumors. Ann Oncol 2004;15:1123-9.
[LinkOut]
- Scagliotti GV, Kortsik C, Dark GG, Price A, Manegold C, Rosell R, et al. Pemetrexed combined with oxaliplatin or carboplatin as first-line treatment in advanced non-small cell lung cancer: a multicenter, randomized, phase II trial. Clin Cancer Res 2005;11:690-6.
[LinkOut]
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1-10.
[LinkOut]
- Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 2006;24:3187-205.
[LinkOut]
- He J, Yang Y, Chen M. Lobectomy by video-assisted thoracoscopic surgery. Zhonghua Wai Ke Za Zhi 1996;34:76-8.
[LinkOut]
- Watanabe A, Koyanagi T, Obama T, Ohsawa H, Mawatari T, Takahashi N, et al. Assessment of node dissection for clinical stage I primary lung cancer by VATS. Eur J Cardiothorac Surg 2005;27:745-52.
[LinkOut]
- Junker K. Histopathologic evaluation of mediastinal lymph nodes in lung cancer. Lung Cancer 2004;45 Suppl 2:S79-83.
[LinkOut]
- Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer 2002;94:2511-6.
[LinkOut]
Cite this article as: He JX, Shao WL, Li SB, Chen MY, Chen HZ, Liu J, Wang W, Qiu Y, Wang DY. Feasibility Of Administering Adjuvant Chemotherapy Of Pemetrexed Followed By Pemetrexed/oxaliplatin Immediately Post -VATS In Patients With Completely Resected NSCLC. J Thorac Dis 2009;1:55-62. doi: 10.3978/j.issn.2072-1439.2009.12.01.002
|




