A cross-sectional and follow-up study of leukopenia in tuberculosis patients: prevalence, risk factors and impact of anti-tuberculosis treatment
Introduction
Tuberculosis is a chronic respiratory infectious disease caused by Mycobacterium tuberculosis. In 2012 there were 8.6 million new cases of tuberculosis and 1.3 million tuberculosis associated deaths worldwide (1), and the corresponding figures in China were 1 million and 44,000, respectively (2). The prevalence of tuberculosis is heterogeneous across different countries and regions; the prevalence is relatively low in developed countries, and 95% of tuberculosis patients are distributed in developing countries (3). China is one of 22 high tuberculosis burden countries around the world, second only to India (4). According to the national fifth epidemiological sampling survey of tuberculosis in 2010 (5), the prevalence of active tuberculosis was 459/1,015 in people 15 years of age and older, and increased with age, suggesting that the burden of tuberculosis is still very high in China.
Anti-tuberculosis drugs can kill and inhibit Mycobacterium tuberculosis, but they also have some side effects on different organs, especially the hematological system (6). Mechanistic studies have shown that anti-tuberculosis drugs such as rifampicin can bind to plasma macromolecular proteins, promote antibody generation, and form antigen-antibody complexes. When these complexes are absorbed on leukocytes, they can cause leukocyte lysis and target cell damage, leading to leukopenia (7-9). In a study by Nagayama et al. in Japan, they found that anti-tuberculosis drugs isoniazid and rifampicin can cause leukopenia (10); Lee et al. in South Korean found that the use of first-line anti-tuberculosis drugs can lead to leukopenia (11). In China, only some case reports are showing that initial treatment with isoniazid, rifampicin, pyrazinamide and ethambutol, rifapentine or levofloxacin could cause leukopenia (12,13). But the researches for the prevalence of and risk factors of leucopenia in tuberculosis patients during the course of therapy are rare.
Patients with leucopenia have potential immunosuppression. The more severe leucopenia could lead to the more bacterial infections (14). So it should be concerned. This cross-sectional study analyzed peripheral blood leukocyte levels in tuberculosis patients, investigated the prevalence of and risk factors for leukopenia in tuberculosis patients, and conducted a follow-up study of tuberculosis cases to explore the influencing factors of leucopenia with an aim to remind clinicians to pay close attention to those who have high prevalence and risk factors when using anti-tuberculosis drugs.
Materials and methods
Subjects
A total of 1,904 tuberculosis outpatients or inpatients who were treated at 11 hospitals in Jiangsu Province, China from March 1, 2013 to May 31, 2013 were included in the study. They ranged in age from 15 to 93 years, with a mean age of 46.6±19.5 years. There were 1,308 (68.7%) males and 596 (31.3%) females. There were 1,440 newly treated cases and 464 previously treated cases. The cases with equal to or higher than normal baseline white blood cell counts were followed up. Of all the newly treated cases, 1,249 completed the follow-up and 41 (3.18%) were lost to follow-up. Newly treated cases were defined as patients who did not receive any anti-tuberculosis therapy or had received anti-tuberculosis therapy for less than one month. Previously treated cases were defined as patients who had received any anti-tuberculosis therapy for at least 1 month, including newly diagnosed cases, recurrent cases and those failing initial treatment. This study was approved by the Ethical Committee of Clinical Research in Nanjing Chest Hospital. All the patients signed written informed consent and then underwent a face-to-face questionnaire survey. The questionnaire covers the following information: basic information including gender, ethnicity, age, resident area, degree of education, income, height, weight, medical insurance, and life habit; diagnosis and treatment information including diagnosis, complications, initial treatment or not, duration of symptoms, diagnosis and treatment processes, and medications; laboratory findings including blood routine examination and liver and kidney function tests; and follow-up information including treatment duration, treatment regimen, blood routine examinations, liver and kidney function tests, and body weights at 2, 4 and 8 weeks of treatment.
Statistical analysis
We first described baseline peripheral blood leukocyte levels in all patients and calculate the percentage of patients with leukopenia (leukocyte count <4.0×109/L). Secondly, we used univariate Logistic regression analysis to identify factors influencing leukopenia in newly treated and previously treated cases. In order to avoid missed factors influencing leukopenia, we chose a level of statistical significance (namely, α=0.1). Statistically, our aim was to reduce the probability of type II errors. Thus, entering variables showing a significant difference (P≤0.10) in univariate analysis into a multivariate Logistic regression model was used to estimate the independent effect of each factor on leukopenia in tuberculosis patients. Then we used univariate COX proportional hazards models to monitor the changes in peripheral blood leukocyte levels of newly treated cases with normal baseline leukocyte levels or higher at 2, 4 and 8 weeks after anti-tuberculosis treatment, to describe the trend of changes in peripheral blood leukocyte levels of tuberculosis patients stratified by demographic characteristics, dietary habits and disease history (non-treatment factors), and to analyze the potential important influencing factors. Finally, to describe the trend of changes in peripheral blood leukocyte levels of tuberculosis patients receiving different treatment regimens, we used covariance analysis to assess the impact of anti-tuberculosis drugs on peripheral blood leukocyte levels. All statistical analyses were conducted using SPSS version 18.0.
Results
A total of 1,904 tuberculosis patients were included in this study. They ranged in age from 15 to 93 years, with a mean age of 46.6±19.5 years. There were 1,308 (68.7%) males and 596 (31.3%) females. Based on diagnostic findings, 35 (1.8%) had primary pulmonary tuberculosis, 23 (1.2%) had disseminated tuberculosis, 1,325 (69.6%) had secondary pulmonary tuberculosis, 334 (17.6%) had pleural tuberculosis, and 187 (9.8%) had other types of extrapulmonary tuberculosis.
Prevalence of and risk factors for leukopenia in tuberculosis patients
This cross-sectional study included 1,440 (75.6%) newly treated tuberculosis patients and 464 (24.4%) previously treated tuberculosis patients. The baseline peripheral blood leukocyte levels of newly treated patients is 6.4±2.6, that of previously treated is 6.6±2.5. And the prevalence of leukopenia in newly treated patients is 150 (10.4%), while that of previously treated tuberculosis patients is 42 (9.1%).
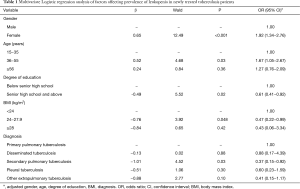
In newly treated tuberculosis patients, univariate Logistic regression analysis showed that being female, advanced age (36−55 years and ≥56 years) and diabetes were risk factors for leukopenia (P<0.10), while secondary pulmonary tuberculosis, higher BMI (24−27.9 kg/m2), higher degree of education (senior high school or above) and longer duration of symptoms (>6 months) were protective factors (P<0.10). Multivariate Logistic regression analysis showed that being female and advanced age (36−55 years) were independent risk factors (P<0.05), while secondary pulmonary tuberculosis, higher BMI (24−27.9 kg/m2) and higher degree of education (senior high school or above) were independent protective factors (P<0.05) (Table 1).
Full table
In previously treated tuberculosis patients, univariate Logistic regression analysis showed that being female, advanced age (≥56 years) and longer duration of previous anti-tuberculosis treatment (>6 months) were risk factors for leukopenia (P<0.10), while smoking and drinking were protective factors (P<0.10), which may be due to the fact that there was a higher proportion of males who had smoking and drinking habits. Multivariate Logistic regression analysis showed that longer duration of previous anti-tuberculosis treatment (>6 months) was an independent risk factor for leukopenia in previously treated tuberculosis patients (P=0.04).
Trend of changes in peripheral blood leukocyte levels of newly treated cases with normal baseline leukocyte levels or higher
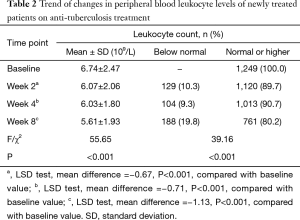
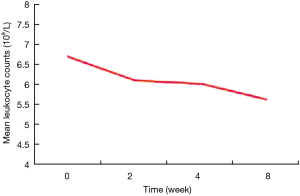
This study included a total of 1,249 newly treated cases with normal baseline leukocyte levels or higher. After 2 weeks of anti-tuberculosis treatment, leukopenia developed in 129 (10.3%) cases. After 4 weeks of anti-tuberculosis treatment, 132 (10.6%) patients were lost to follow-up, and 104 (9.3%) patients developed leukopenia. After 8 weeks of anti-tuberculosis treatment, 168 (15.0%) patients were lost to follow-up, and 188 (19.8%) patients developed leukopenia. With the prolongation of treatment duration, the percentage of tuberculosis patients with leukopenia gradually increased, while peripheral blood leukocyte levels gradually declined (P<0.001) (Table 2, Figure 1).
Full table
Trend of changes in peripheral blood leukocyte levels of tuberculosis patients stratified by non-treatment factors
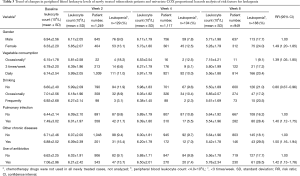
Using treatment duration as a time variable (2, 4 and 8 weeks of treatment), leukopenia (white blood cell count <4.0×109/L) as an outcome variable, and demographic characteristics, dietary habits, as well as disease related factors as independent variables, we analyzed 1,249 newly treated cases using univariate COX proportional hazards models. The results showed that gender, vegetable consumption, drinking, pulmonary infection, other chronic diseases, and use of antibiotic were significantly associated with the development of leukopenia in tuberculosis patients after anti-tuberculosis treatment (P<0.05), suggesting that the above factors should be controlled when analyzing the impact of use of anti-tuberculosis drugs on leukopenia in tuberculosis patients (Table 3).
Full table
Impact of anti-tuberculosis drugs on the trend of changes in peripheral blood leukocyte levels of tuberculosis patients
Of 1,249 newly treated tuberculosis patients, 882 (70.6%) were treated with first-line anti-tuberculosis drugs, mainly 2−3HRZE(S)/4−10HR(E) chemotherapy regimens; 200 (16.0%) were treated with first-line anti-tuberculosis drugs combined with second-line drugs, namely, first-line treatment regimens combined with second-line drugs such as 4-aminosalicylic acid, amikacin, ofloxacin or levofloxacin; only 8 (0.7%) were treated with second-line anti-tuberculosis drugs (due to the number of cases was too small, they were not included in the data analysis); and another 159 (12.7%) were treated with unknown drugs, who were not included in the data analysis. The peripheral blood leukocyte levels of tuberculosis patients treated with different regimens at different time points after treatment, and the trend of leukocyte changes are shown in Tables 4 and 5. [Note: 2−3HRZE(S)/4−10HR(E): isoniazid, rifampicin, pyrazinamide, ethambutol with or without streptomycin for 2−3 months, then isoniazid, rifampicin with or without ethambutol for 4−10 months].

Full table

Full table
As shown in Table 3, unevenly distributed factors such as gender, dietary habits, and co-morbidities had a significant effect on peripheral blood leukocyte levels of tuberculosis patients. After controlling the above factors using covariance analysis, we assessed the independent effect of anti-tuberculosis therapy on peripheral blood leukocyte levels of tuberculosis patients, and estimated the adjusted mean peripheral blood leukocyte counts at different time points a different treatment.
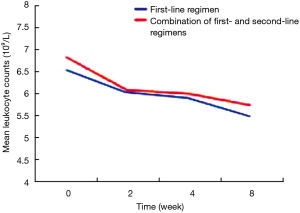
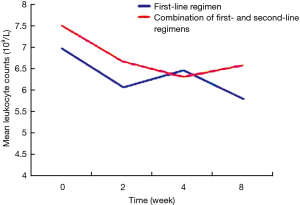
The patients were divided into two groups based on the use of antibiotics or not. According to the results of analysis of covariance, after controlling for gender, drinking, vegetable consumption, pulmonary infection, other chronic diseases, and baseline levels of peripheral blood leukocytes, peripheral blood leukocyte counts in patients receiving different treatments did not differ significantly at 2, 4 and 8 weeks (P>0.05), suggesting that different anti-tuberculosis regimens, namely first-line regiments and first-line regiments combined with second-line drugs, had no statistically significant effect on the levels of peripheral blood leukocytes (Table 5). On the other hand, based on the adjusted mean peripheral blood leukocyte counts at different time points estimated by covariance regression analysis, it was found that in patients without use of antibiotics, peripheral blood leukocyte levels showed a gradually declining trend with the prolongation of the use of anti-tuberculosis drugs (P<0.001), while in patients with use of antibiotics, peripheral blood leukocyte levels showed a decline trend initially, but decreased slow or slightly rebounded subsequently, although they were still below the baseline levels (Figures 2 and 3).
Discussion
Currently, the burden of tuberculosis is high in China, where the disease is characterized by a high prevalence, high rate of drug resistance, high mortality, and high morbidity (15). Anti-tuberculosis drugs achieve the goal of treatment of tuberculosis by altering the basic biological process after entering into the human body, but they often result in some adverse reactions, including those affecting the hematological system, digestive system, kidney and liver function and the central nervous system (15), with the hematological system being one of the most commonly affected systems (6). The present study analyzed the characteristics of tuberculosis patients with leukopenia by investigating the prevalence of and risk factors for leukopenia in tuberculosis patients, and explored the impact of anti-tuberculosis drugs on peripheral blood leukocyte counts of tuberculosis patients by conducting a follow-up study of newly treated tuberculosis patients and previously treated tuberculosis patients, thus providing a basis finding for clinicians to be cautious when facing the patients with high risk of leucopenia during the therapy course.
Characteristics of tuberculosis patients with leukopenia
This study found that in newly treated tuberculosis patients, the prevalence of leukopenia was higher in women, which is consistent with the finding by Lee et al. (11). This may be because women’s immune function is lower than men’s. In addition, the prevalence of leukopenia was higher in older patients, which may be associated with decreased immune function in the elderly (16). These findings suggest that special attention should be paid to hematological system and immune system functions of female and elderly tuberculosis patients in the diagnosis and treatment of tuberculosis.
We found that the prevalence of leukopenia in patients with higher education degree was lower, which may be because such patients had good health and lifestyle habits and more exercise that could enhance their immunity. This suggests that patients with tuberculosis should cultivate good health and lifestyle habits and enhance exercise to improve their immunity. Patients who accept high degree of education indicates more understanding and compliance of the disease. Otherwise, reasonable diet structure may be factor of avoiding leucopenia. Also, the prevalence of leukopenia in patients with a higher BMI was lower. Higher BMI is often associated with relatively abundant intake of nutrients, and studies have found that patients with higher protein contents have elevated levels of CD4+ T lymphocytes, which can enhance the immune function through secretion of lymphatic factors (17), while the vegetable diet was associated with an increased risk of leukopenia. The reason might be that the patients prefer a vegetarian diet may take less fat and protein, which will cause the lack of feedstock of blood. In addition, we found that the prevalence of leukopenia in patients with secondary pulmonary tuberculosis was lower. This may be because T cell-mediated immune memory responses to secondary tuberculosis infection were faster than immune responses to primary tuberculosis infection (15).
In addition, this study found that the prevalence of leukopenia in previously treated patients with longer previous anti-tuberculosis treatment duration (>6 months) was higher, which is consistent with the previous finding (18). In such patients, immune system function often gradually declines, and the ability to defend and remove pathogenic agents is weakened. As a result, pulmonary complications such as infection often occur, which can lead to reduced leukocyte counts.
Impact of anti-tuberculosis drugs on peripheral blood leukocyte counts of tuberculosis patients
In tuberculosis patients treated with anti-tuberculosis regimens not containing antibiotics, the prevalence of leukopenia gradually increased and peripheral blood leukocyte levels gradually declined with the prolongation of treatment duration. After controlling for gender, drinking, vegetable consumption, pulmonary infection, other chronic diseases, and baseline levels of peripheral blood leukocytes, peripheral blood leukocyte counts in patients receiving different treatments did not differ significantly at different time points, suggesting that both first-line regiments and first-line regiments combined with second-line drugs had no statistically significant effect on the levels of peripheral blood leukocytes at different time points. However, the levels of peripheral blood leukocytes showed a gradually declining trend in patients receiving any of the treatment regimens. Mechanistic studies have shown that anti-tuberculosis drugs as haptens are attached to the surface of blood leukocytes, form complexes, and induce the generation of anti-complex antibodies, whose major parts can be engulfed by macrophages. Anti-tuberculosis drugs can also bind to plasma macromolecular proteins, promote antibody generation, and form antigen-antibody complexes. When these complexes are absorbed on leukocytes, they can cause leukocyte lysis and target cell damage, leading to leukopenia (7,11). The results of our study also demonstrate that anti-tuberculosis drugs can cause leukopenia in tuberculosis patients, and peripheral blood leukocytes show a declining trend with the prolongation of anti-tuberculosis treatment duration.
In tuberculosis patients treated with anti-tuberculosis regimens containing antibiotics, the prevalence of leukopenia gradually increased and peripheral blood leukocyte levels showed a declining trend in patients treated with first-line regimens, but the decline in peripheral blood leukocyte levels was not obvious in patients treated with first-line regimens combined with second-line anti-tuberculosis drugs. After controlling for other non-treatment factors, the impact of different treatments on peripheral blood leukocyte counts at different time points showed no significant differences, and with the prolongation of treatment duration, peripheral blood leukocyte counts decreased slow or slightly rebounded. The possible explanation is that antibiotic treatment initially leads to a pronounced decrease in leukocyte count. The use of antibiotics improves the ability of macrophages to engulf bacteria, enhances the bactericidal effect of polymorphonuclear leukocytes and macrophages and thereby improves the coordinated bactericidal effects of the immune system (19). As a result, peripheral blood leukocyte counts decreased slow or slightly rebounded.
Conclusions
To sum up, female patients, patients at advanced age and recurrent tuberculosis patients having longer previous anti-tuberculosis treatment are high-risk populations for leukopenia, while those with higher education level, higher BMI, and secondary pulmonary tuberculosis are associated with a lower prevalence of leukopenia. Anti-tuberculosis drugs can affect the occurrence and development of leukopenia, while patients’ living habits such as vegetable consumption and drinking, co-morbidities and the use of antibiotics may also affect the development of leukopenia. These findings suggest that clinicians should pay attention to various influencing factors during anti-tuberculosis treatment so as to be able to promptly find the changes of the hematological system and give timely symptomatic treatment to guarantee standard anti-tuberculosis treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization. Global Tuberculosis Report 2013:1-289. Available online: http://www.who.int/tb/publications/global_report/en/, [accessed on 2014-8-15].
- World Health Organization. World Health Statistics 2012:1-180. Available online: http://www.who.int/gho/publications/world_health_statistics/2012/en/, [access on 2014-8-15].
- Peng W, Wang Y, Xiao C. New Edition of Tuberculosis, Second Edition. Beijing: Chinese Medicine and Technology Publishing House 2003:139-60. [In Chinese].
- Editorial Committee of Guideline of Implementation of Tuberculosis Prevention and Treatment Program in China. Guideline of Implementation of Tuberculosis Prevention and Treatment Program in China. Beijing: Peking Union Medical College Press 2009:1-7. [In Chinese].
- Technical Guidance Group of the Fifth National TB Epidemiological Survey, The Office of the Fifth National TB Epidemiological Survey. The fifth national tuberculosis epidemiological survey in 2010. Chinese Journal of Antituberculosis 2012;34:485-508. [In Chinese].
- Zhang DR. Modern Phthisiology. Beijing: People's Military Medical Press 2000:604-7. [In Chinese].
- De Vriese AS, Robbrecht DL, Vanholder RC, et al. Rifampicin-associated acute renal failure: pathophysiologic, immunologic, and clinical features. Am J Kidney Dis 1998;31:108-15. [PubMed]
- Rekha VV, Santha T, Jawahar MS. Rifampicin-induced renal toxicity during retreatment of patients with pulmonary tuberculosis. J Assoc Physicians India 2005;53:811-3. [PubMed]
- Jover-Sáenz A, Porcel-Pérez JM, Madroñero-Vuelta AB, et al. Acute interstitial nephritis due to rifampicin. Enferm Infecc Microbiol Clin 2006;24:64. [PubMed]
- Nagayama N, Shishido Y, Masuda K, et al. Leukopenia due to anti-tuberculous chemotherapy including rifampicin and isoniazid. Kekkaku 2004;79:341-8. [PubMed]
- Lee SW, Lee SM, Yoo CG, et al. Leukopenia during treatment with first-line anti-tuberculosis medication. Respiration 2005;72:660-1. [PubMed]
- Zhu M, Liu YD, Zhao Q, et al. Hematological system abnormalities caused by anti-tuberculosis drugs. Chinese Journal of Antituberculosis 2004;26:338-40. [In Chinese].
- Wang Q, Chen JH, Han Q, et al. Adverse drug reactions in hematological system induced by anti-tuberculosis drugs: literature review of 441 cases. China Pharmacy 2008;19:376-7. [In Chinese].
- Czajkowska M, Augustynowicz-Kopeć E, Zwolska Z, et al. Pulmonary mycobacterioses--frequency of occurrence, clinical spectrum and predisposing factors. Pneumonol Alergol Pol 2002;70:550-60. [PubMed]
- Ma Y, Zhu LZ, Pan YX. Tuberculosis. Beijing: People's Health Publishing House 2006:1-622. [In Chinese].
- Kawai K, Meydani SN, Urassa W, et al. Micronutrient supplementation and T cell-mediated immune responses in patients with tuberculosis in Tanzania. Epidemiol Infect 2014;142:1505-9. [PubMed]
- Cahn P, Ruxrungtham K, Gazzard B, et al. The immunomodulatory nutritional intervention NR100157 reduced CD4+ T-cell decline and immune activation: a 1-year multicenter randomized controlled double-blind trial in HIV-infected persons not receiving antiretroviral therapy (The BITE Study). Clin Infect Dis 2013;57:139-46. [PubMed]
- Han O, Li S. Clinical analysis of 164 cases of previously treated tuberculosis with pulmonary infection. Chinese Community Doctors 2012;14:159-60. [In Chinese].
- von Rosenvinge EC, Song Y, White JR, et al. Immune status, antibiotic medication and pH are associated with changes in the stomach fluid microbiota. ISME J 2013;7:1354-66. [PubMed]


