Transthoracic needle aspiration: the past, present and future
Introduction
From as early as 1883 (1), clinicians have used transthoracic needle aspiration (TTNA) to safely and effectively diagnose pulmonary malignancies. Over the years, biopsies through this procedure have become more accurate and efficient due to advances in imaging technologies and cytopathologic techniques. The transition from fluoroscopy to computed tomography (CT) allowed for better visualization and detection of smaller lesions. In addition, developments in cytopathology have facilitated histologic analysis with smaller amounts of required material. Previous studies have shown diagnostic accuracy of benign and malignant disease of TTNA to range from 77-97% (2-4). Increased use of CT-guided TTNA (CT-TTNA) has reduced the need for more invasive surgeries such as thoracotomies and bronchoscopies. As a result, there has been increases in cost savings and decreases in hospital length of stay (2). However, there are few complications that arise from TTNA, pneumothorax being the most common. When making the decision to do TTNA for a biopsy, physicians need to consider from the givens of TTNA and the patient whether the benefits of the biopsy greatly outweigh the risk and consequence of a complication. In this review article, we will discuss what is known about indications, contraindications, imaging modalities, needle types, considerations, complications and management with respect to TTNA, and then comment on the trajectory of TTNA in the future of thoracic medicine.
Indications
TTNA is mostly used to evaluate an indeterminate pulmonary mass or nodule. This can include almost any abnormality seen in imaging. Lesions of lung parenchyma, mediastinal, hilar, pleural, and chest wall have been proven to be diagnosable with TTNA (1). In these cases, some institutions would instead use bronchoscopy or thoracoscopic surgery. However, bronchoscopy is limited to central lesions with endobronchial or hilar components (3,5) and thoracoscopic surgery requires general anesthesia and is dependent on the skill of the surgeon (3). In addition, TTNA can be used to sample focal infectious lesions and stage lung cancers or extrathoracic malignancies (3). For these cases, a histological classification would be very useful, enhancing the value of TTNA. Besides neoplastic (benign or malignant) disease, TTNA can be used to diagnose inflammatory disease (1).
Contraindications
The number one contraindication to TTNA is bleeding diathesis, for which platelet count, prothrombin time, partial thromboplastin time and INR should be obtained prior to procedure. Relative contraindications range from pulmonary hypertension to emphysematous disease and chronic obstructive pulmonary disease (COPD). Large bullae in biopsy path can cause post-procedure pneumothorax. Possibly the most serious contraindication to TTNA is an uncooperative patient. The patient’s ability to lie still, hold their breath, and maintain prone position (1,3) are essential to a successful TTNA. For this reason intractable cough and mechanical ventilation are also contraindications.
Imaging modalities
Fluoroscopy
Fluoroscopic guidance represented the first modality for TTNA (3). Advantages of fluoroscopy include real time visualization, familiarity, and availability. Fluoroscopy-guided TTNA (F-TTNA) has been an alternative to CT-TTNA due to shorter durations, lower incidence of pneumothorax, lower cost and lower radiation dosage (3,6). Disadvantages arise from limitations of fluoroscopy, including poor visualization of small lesions and bullae and safe sampling of central lesions. One study done by Cheung et al. (6) investigated combining F-TTNA with CT-TTNA, where a biopsy is done under fluoroscopy and cone-beam CT using a C-arm cone beam CT system. This new biopsy technique took the advantages of fluoroscopy and CT and allowed for diagnosis of small (<30 mm) and deep (≥50 mm) with high diagnostic accuracy and short procedure time (6).
Ultrasound
Ultrasound-guided TTNA biopsy has many advantages, in that it provides real-time visualization, can target nonvascular and nonnecrotic portions of masses (7), and has no radiation. Compared to CT-TTNA, ultrasound is inexpensive and portable. The latter aspect is useful for patients who cannot be transported to the radiology department (3). However, the necessity of adequate acoustics limits the potential of using ultrasound. Masses in the anterior mediastinum and peripheral lung adjacent to the costal pleural surface are difficult to image in ultrasound, but are detectable by CT (3,8).
Magnetic-resonance imaging (MRI)
Magnetic-resonance imaging (MRI)-guided procedures have been shown to yield excellent clinical results in osseous biopsies (9), however very little has been reported on MRI usage in TTNA (10,11). This might be because CT is generally accepted as the main imaging modality for TTNA. Liu et al. (10) showed that MRI-guided TTNA (MRI-TTNA) is comparable to CT in mean procedural time and diagnostic accuracy. Sakarya et al. (11), who first reported using an MR fluoroscopy-guided approach to TTNA, showed that MR guidance has good feasibility. Advantages of using MR fluoroscopy include near-real-time imagine and absence of radiation (11). However, with CT imaging, fissures, focal areas of emphysema, and bullae are better detected and during TTNA these areas can be avoided during biopsy (11). This is something that MR imaging cannot account for. In addition, issues with breath-hold duration and patient compliance can create motion artifacts, which means lower MR-image quality.
Computed tomography (CT)
As previously mentioned, CT is the most common modality for TTNA procedures. Advantages of CT guidance are attributed to excellent visualization capabilities. This also includes the visualization of the biopsy needle tip, which facilitates placement and allows for high diagnostic yield (3). However, there are disadvantages, including lack of real-time visualization, greater cost of procedure, more patient discomfort, and higher incidence of pneumothorax (3). Within this modality, there have been many developments. First, conventional CT was used to guide biopsy needle placement. Soon, helical CT emerged and now is very widely used (12). CT fluoroscopy was most recently introduced, and has been proven to be effective because it unites together the visualization of CT and efficiency from fluoroscopic guidance. Unfortunately, this technique is susceptible to irradiation to the physician’s hand during the procedure. Yoshimatsu et al. (12) conducted a TTNA study using an “I-I device” which functions as a cradle for the needle and a handle to distance a physician’s hand from radiation exposure. Results of this study showed decreased radiation dose to physician’s hand and faster fluoroscopy and procedure times with equal if not better outcomes than those without the I-I device (12). Surgical forceps or special needle holders are currently used to reduce radiation dose during TTNA procedures (12).
CT-TTNA has been improved upon through the incorporation of other technologies and modalities. About 10 years ago, cone-beam CT had been introduced to interventional radiology as cutting-edge, allowing for real-time fluoroscopy, angiography, CT imaging, and 3D reconstruction capabilities (13). Previous studies have shown high and reproducible diagnostic accuracy with cone-beam CT-TTNA (13,14). Cone-beam CT has even been shown to provide high accuracy, specificity, sensitivity, and positive/negative predictive values with “XperGuide” navigation guidance (15). One group had even combined fludeoxyglucose positive emission tomography (FDG-PET) with CT, and showed improved diagnostic yield in patients with large (≥2.5 cm) intrathoracic lesions (16). Another group had assessed performance of using an optical CT-based navigation system to assist with TTNA. With a technical success of 100%, Grasso et al. (17) showed that using this navigation tool with a low dose CT protocol can decrease patient radiation exposure.
Navigation systems
Navigation systems for assisting with TTNA have been shown to be effective in CT and electromagnetic modalities. Electromagnetic navigation bronchoscopy (ENB) has recently started to emerge, and has been quite effective (18,19). ENB uses an image guided localization system that directs steerable bronchoscopic tools to points of interest within a bronchial tree, and has been shown to produce high sensitivity in fine-needle aspiration (19). Electromagnetic Navigational TTNA (E-TTNA) is currently being proposed to maximize diagnostic yield and minimize the need for invasive procedures in conjunction with endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) (18). Another type of navigation system seen in literature uses virtual assisted lung mapping (VAL-MAP) to provide “geometric” information of the lung surface (20). Bronchoscopic multispot dye-marking was used to produce markings that can be visualized through CT in order to produce 3D virtual images for reference. VAL-MAP was shown in an early experience study to work, but more studies need to be done to assess its safety and efficacy (20).
Needles
There are many types of needs used in TTNA for biopsies, with various gauges, lengths, tips, and mechanisms. A clinician may select a needle based on preference or patient/lesion characteristics. Mainly, there are aspiration needles, cutting needles, and automated core-biopsy needles. Aspiration needles are thin-walled and flexible (2) and can provide high-quality cellular material for microscopic/cytological diagnosis (3). The Chiba (Cook, Inc. Bloomington, IN, USA) is the most commonly used, and has a 30 degree bevel and sizes ranging from 18-25 gauge (2). This type of needle can be used to diagnose epithelial carcinomas. Cutting needles are used when a histologic specimen is required for analysis. Essentially, cutting needles are modified aspiration needles, with an added cutting tip on the side. Possible cutting tips include a receptacle slot just proximal to the tip, a spring-loaded end cutting tip, or a drill bit styled tip within a guiding cannula (3). Lastly, automated core-biopsy needles (Figure 1) retrieve tissue from the patient for histopathologic workup. These needles are useful especially if a pathologist is not on-site, because the needle will obtain similar core samples that are free of crush injury sometimes imparted by smaller conventional needles (2).
Considerations
The primary consideration a physician must make before pursuing TTNA for a patient is whether the benefits of a biopsy outweigh the risk for complication and toleration of the procedure. Pneumothorax remains the most common complication during and after a TTNA procedure (2,3,21). Many previous studies investigating this connection have proven factors that contribute to this increased rate of complication. Geraghty et al. (22) showed needle size and patient age to be statistically significant risk factors. In the same study, they measured a 50% reduction in pneumothorax rate associated with using smaller 19-gauge needles instead of 18-gage needles (22). Patients with larger lesions are susceptible to higher pneumothorax rate (23,24) and lower diagnostic accuracy during TTNA (4). Longer needle path lengths in procedure are also significant in increasing pneumothorax rate and decreasing diagnostic accuracy (4). Interestingly, the risk of pneumothorax in TTNA is not dependent on physician experience (23) or needle aspiration technique (2). In one study, pneumothorax incidence was nearly equal in TTNA performed by a junior resident, senior resident, or staff physician (23).
Overall, patients who are female, older age (≥70 years), have COPD (25), and/or have presence of emphysema (23) are at higher risk for complications during TTNA. As more and more patients are being diagnosed with thoracic malignancy and undergo TTNA for biopsy, the incidence of complication will definitely increase, especially because smaller growing lesions (<20 mm) can now be detected. Patient lifestyle, age, comorbidities, and experiences at an institution must be taken into consideration before electing for TTNA.
Complications and management
Pneumothorax
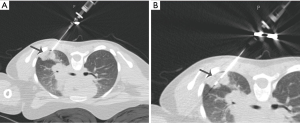
As previously mentioned, pneumothorax is the most common complication as a result of TTNA. The incidence of pneumothorax has been reported to be from 0-61%, with an average of around 20% (2,3,21-23). Increased patient age, obstructive lung disease, COPD, multiple pleural passes, increased duration of procedure, and traversal of a fissure increases the risk of pneumothorax. If a pneumothorax develops during procedure, it can be manually aspirated before the introducer needle is removed (Figure 2) (21). It is also recommended that the patient be placed biopsy-side down and given oxygen via nasal cannula. For pneumothoraces that develop after procedure, many steps need to be taken. Small pneumothoraces can be managed with monitoring of vital signs, administration of oxygen, and obtaining a chest film. If films at 2 and 4 hours are unremarkable, discharge can be considered. Pneumothoraces unchanged on film after 4 hours are unlikely to grow (2,26). Larger cases might require image-guided intervention such as insertion of a small chest tube, which happens for around 1-14.2% of patients (3,21). These small chest tubes allow for treatment of potentially significant complication without the need for surgical intervention and can be inserted on an outpatient basis. In addition, the diagnostic efficacy increases for biopsies of lesions with an already treated pneumothorax (1).

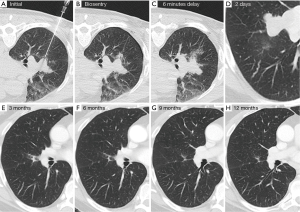
Most recently, lung biopsy tract seal plugs have been used to combat pneumothorax (Figure 3). Two major types are the Bio-Seal Lung Plug System (Angiotech Pharmaceuticals, Vancouver, British Columbia) and the BioSentry Tract Sealant System (Surgical Specialties, Massachusetts, USA). One study done by Zaetta et al. (27) showed significantly reduced rates of pneumothorax in patients undergoing CT-TTNA. Autologous blood clot seals (ABCS) have also been shown to reduce frequency of pneumothoraces (28).
Hemorrhage and hemoptysis
The second most common complication with TTNA is hemorrhage, with an average incidence of about 11% (2,3,21). Hemorrhages and hemoptysis are considered self-limited and usually do not require intervention. However, the patient should be placed biopsy-side down to prevent aspiration of blood into the opposite lung (2,3,21). Fortunately, many measures can be taken to avoid a hemorrhage or hemothorax. Patients taking anticoagulants or have bleeding diatheses should be contraindicated for TTNA. During the procedure, it is recommended to aim the trajectory of the needle away from the aorta and heart to avoid accidental injury. Massive hemorrhages are very rare, but may require anesthesia consultation to intubate with a double-lumen endotracheal tube (21).
Air embolism
Air embolism in TTNA is extremely rare but can be very fatal if not fixed, leading to cerebral or myocardial infarction, stroke, or death. As little as 0.5 mL of air would be required to induce coronary ischemia or arrhythmias (3). In large cohorts of TTNA patients, there were very few cases of air embolism (occurrence between 0.02-1.8%) (2,21). There are two mechanisms by which air embolisms can occur: (I) placement of the needle tip in a pulmonary vein and removal of the inner stylet and (II) placement of the needle through both a bronchus and adjacent pulmonary vein, followed by creation of a fistula. If an air embolism is recognized, the patient should be placed in the Trendelenburg position to prevent air from being circulated cerebrally (21). Air embolisms can be prevented by occluding the introducer needle with an inner stylet, saline, or a finger. This complication is another reason why patients with intractable coughs should not be recommended for TTNA.
Tumor seeding
Tumor seeding is an extremely rare complication of TTNA, and is reported to have incidence between 0.012-0.061% (3,21,29). Mean time from biopsy to development of metastasis is around 2.6 months (3). At this time, there are no definitive risk factors that can be identified to help prevent tumor seeding (21,29). However, if metastasis occurs but is isolated to the chest wall, wide en bloc resection can be done. Tumor seeding has occurred with diagnoses of pleural mesothelioma and thymoma.
Future perspectives
After so many years of implementation and research, TTNA has been proven to be safe, effective, and very accurate in biopsy diagnosis. Advances in imaging and cytopathology have increased diagnostic power of TTNA, meaning that more lesions (including those previously too small to detect) can be analyzed with less required histologic material. However, because of this the number of diagnoses has and will increase over time. This will inevitably increase complications, which means that there will be more weight on the decision to elect a patient for TTNA. In this case, more studies into risk factors need to be done, especially for complications not as well researched as pneumothoraces. A very key aspect of TTNA is the length of procedure, which has been shown to be a significant risk factor for complication. Future studies on needle type and design may open the door to technologies that will make the procedures more efficient and decrease patient length of stay. Results from a TTNA biopsy will also pave way to a form of personalized medicine. Tissue and cells retrieved during TTNA can be placed through genetic blood testing in order to specifically identify biomarkers and provide tailor-made treatment for each specific patient. These considerations and the prospective trajectory for the field can only increase the safety, efficacy, and acceptance of TTNA as the mainstay procedure to diagnose thoracic malignancy and disease.
Acknowledgements
CT images for Figure 3 were given to us by Robert Suh, MD (UCLA Medical Center, Diagnostic Radiology).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Perlmutt LM, Johnston WW, Dunnick NR. Percutaneous transthoracic needle aspiration: a review. AJR Am J Roentgenol 1989;152:451-5. [PubMed]
- Birchard KR. Transthoracic needle biopsy. Semin Intervent Radiol 2011;28:87-97. [PubMed]
- Klein JS, Zarka MA. Transthoracic needle biopsy. Radiol Clin North Am 2000;38:235-66. vii. [PubMed]
- Ohno Y, Hatabu H, Takenaka D, et al. CT-guided transthoracic needle aspiration biopsy of small (< or = 20 mm) solitary pulmonary nodules. AJR Am J Roentgenol 2003;180:1665-9. [PubMed]
- Naidich DP, Sussman R, Kutcher WL, et al. Solitary pulmonary nodules. CT-bronchoscopic correlation. Chest 1988;93:595-8. [PubMed]
- Cheung JY, Kim Y, Shim SS, et al. Combined fluoroscopy- and CT-guided transthoracic needle biopsy using a C-arm cone-beam CT system: comparison with fluoroscopy-guided biopsy. Korean J Radiol 2011;12:89-96. [PubMed]
- Pan JF, Yang PC, Chang DB, et al. Needle aspiration biopsy of malignant lung masses with necrotic centers. Improved sensitivity with ultrasonic guidance. Chest 1993;103:1452-6. [PubMed]
- Perlmutt LM, Braun SD, Newman GE, et al. Timing of chest film follow-up after transthoracic needle aspiration. AJR Am J Roentgenol 1986;146:1049-50. [PubMed]
- Fritz J, Tzaribachev N, Thomas C, et al. Magnetic resonance imaging-guided osseous biopsy in children with chronic recurrent multifocal osteomyelitis. Cardiovasc Intervent Radiol 2012;35:146-53. [PubMed]
- Liu S, Li C, Yu X, et al. Diagnostic accuracy of MRI-guided percutaneous transthoracic needle biopsy of solitary pulmonary nodules. Cardiovasc Intervent Radiol 2015;38:416-21. [PubMed]
- Sakarya ME, Unal O, Ozbay B, et al. MR fluoroscopy-guided transthoracic fine-needle aspiration biopsy: feasibility. Radiology 2003;228:589-92. [PubMed]
- Yoshimatsu R, Yamagami T, Kato T, et al. Percutaneous needle biopsy of lung nodules under CT fluoroscopic guidance with use of the “I-I device”. Br J Radiol 2008;81:107-12. [PubMed]
- Choi JW, Park CM, Goo JM, et al. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of small (≤20 mm) lung nodules: diagnostic accuracy and complications in 161 patients. AJR Am J Roentgenol 2012;199:W322-30. [PubMed]
- Hwang HS, Chung MJ, Lee JW, et al. C-arm cone-beam CT-guided percutaneous transthoracic lung biopsy: usefulness in evaluation of small pulmonary nodules. AJR Am J Roentgenol 2010;195:W400-7. [PubMed]
- Floridi C, Muollo A, Fontana F, et al. C-arm cone-beam computed tomography needle path overlay for percutaneous biopsy of pulmonary nodules. Radiol Med 2014;119:820-7. [PubMed]
- Sauter J, Butnor K, Klein J. Diagnostic Yield of CT-guided Transthoracic Needle Aspiration of Large Pulmonary Masses is Improved by Pre-procedure FDG-PET Imaging. Journal of American Society of Cytopathology 2014;5:S4.
- Grasso RF, Cazzato RL, Luppi G, et al. Percutaneous lung biopsies: performance of an optical CT-based navigation system with a low-dose protocol. Eur Radiol 2013;23:3071-6. [PubMed]
- Arias S, Lee H, Semaan R, et al. Use of Electromagnetic Navigational Transthoracic Needle Aspiration (E-TTNA) for Sampling of Lung Nodules. J Vis Exp 2015.e52723. [PubMed]
- Odronic SI, Gildea TR, Chute DJ. Electromagnetic navigation bronchoscopy-guided fine needle aspiration for the diagnosis of lung lesions. Diagn Cytopathol 2014;42:1045-50. [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [PubMed]
- Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol 2011;196:W678-82. [PubMed]
- Geraghty PR, Kee ST, McFarlane G, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology 2003;229:475-81. [PubMed]
- Cox JE, Chiles C, McManus CM, et al. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology 1999;212:165-8. [PubMed]
- Kazerooni EA, Lim FT, Mikhail A, et al. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology 1996;198:371-5. [PubMed]
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [PubMed]
- Choi CM, Um SW, Yoo CG, et al. Incidence and risk factors of delayed pneumothorax after transthoracic needle biopsy of the lung. Chest 2004;126:1516-21. [PubMed]
- Zaetta JM, Licht MO, Fisher JS, et al. A lung biopsy tract plug for reduction of postbiopsy pneumothorax and other complications: results of a prospective, multicenter, randomized, controlled clinical study. J Vasc Interv Radiol 2010;21:1235-43.e1-3.
- Lang EK, Ghavami R, Schreiner VC, et al. Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology 2000;216:93-6. [PubMed]
- Robertson EG, Baxter G. Tumour seeding following percutaneous needle biopsy: the real story! Clin Radiol 2011;66:1007-14. [PubMed]


