The role of speckle tracking echocardiography in assessment of lipopolysaccharide-induced myocardial dysfunction in mice
Introduction
Septic cardiomyopathy is a well-described complication of severe sepsis and septic shock. Sepsis-induced myocardial dysfunction is one of the major predictors of morbidity and mortality of sepsis. It is present in more than 40% of patients with sepsis and its appearance can raise the mortality rate up to 70% (1,2). Nearly 30 years of research on septic cardiomyopathy have not been sufficient for improving its prognosis. There are controversies about the pathophysiology of sepsis-induced myocardial depression and its treatment strategies, many of which are still in the experimental period. The Clowes group in 1966, after examination of a cohort of patients with diffuse visceral fulminating peritonitis, suggested that cardiovascular involvement associated with sepsis was based on two patterns (3). Initially, patients had a hyperdynamic phase (“warm shock”), which exhibited hot extremities with a bounding pulse (elevated cardiac output and decreased systemic vascular resistance). Later, the hypodynamic stage occurred (“cold shock”), in which patients had a thready pulse, signs of peripheral hypoperfusion, organ failure and ultimately leaded to death (systemic vascular resistance increased to compensate for reduction of cardiac output) (4). Experimentally, the administration of lipopolysaccharide (LPS) to laboratory animals, especially mice, has been widely used to study the mechanisms of septic cardiomyopathy (5). Endotoxin-induced septic mouse models provide insights about specific components of the septic process but cannot truly mimic the full clinical complexity and intrinsic heterogeneity of patients in sepsis (6). Despite these limitations, mouse models will remain essential in the development of novel therapies for sepsis and septic shock because they provide fundamental information about the pharmacokinetics, toxicity, and mechanism of drug action that cannot be duplicated by other methods (7).
Echocardiography is non-invasive, widely available, cost-effective, and involves relatively short image acquisition and post-processing time. Despite these advantages, conventional echocardiographic measures often underestimate the severity of septic cardiomyopathy because all reference values are based on normal systemic vascular resistance—a prerequisite which is not present in patients with severe sepsis and vasodilation. Furthermore, conventional echocardiography may even mask cardiac impairment due to severe reduction of afterload in septic shock (8-10). Recently, speckle tracking echocardiography (STE), a novel echocardiographic imaging technique based on myocardial strain analysis, has been found to dramatically improve the assessment of left ventricular (LV) performance in humans (11). By capturing segmental tissue motion in multiple planes and axes serially over the cardiac cycle, strain analysis provides integrated and detailed information regarding both regional and global LV function with much greater sensitivity and specificity than conventional echocardiographic measurements, including fractional shortening (FS) or ejection fraction (EF) (12). Recent studies showed that LV strain and torsion by STE were impaired in septic patients (13,14).
For the reasons given above, we hypothesize that STE is a reliable method to assess LPS-induced cardiac dysfunction in mouse models, which may be beneficial for the diagnosis and prognosis of septic cardiomyopathy.
Methods
Animals
Male C57BL/6J mice (8–10 weeks old) weighing 23–26 g were obtained from the Nanjing University Model Animal Research Center. The newly arrived animals were kept at least 10 days in a 12 h/12 h light/dark cycle at 21–23 °C and fed a standard chow diet ad libitum before experiments. Before and during experiments, body temperature was determined by measuring the rectal temperature at 1.5 cm depth with a temperature probe THM 150 (Indus Instruments, Houston, USA). Mice were sacrificed and blood was immediately collected from the retro-orbital sinus. The blood was allowed to clot at room temperature for 10 min, serum was obtained by centrifugation (approximately 1,200 ×g for 10 min at 4 °C), and stored at −80 °C until use. All procedures described in this study were approved by the Nanjing Medical Unversity Committee on Animal Care. All experiments were performed with the guidelines for the “Principles of Laboratory Animal Care” and the “Guide for the care and use of laboratory animals” published by the National Institutes of Health.
Lipopolysaccharide (LPS) injection
E. coli LPS (serotype O111:B4, Sigma-Aldrich, St Louis, MO, USA), was dissolved in sterile physiological saline (0.9% NaCl) at a concentration of 1 mg/mL. Mice were injected intraperitoneally with 10 mg of LPS per kg body weight.
Experimental protocol
2-D echocardiographic LV short-axis images were assessed in this study. FS and EF were measured from standard M-mode tracings, whereas Scirc and Srad were derived offline by STE using Echopac PC software (Version 12.0.0, GE Vingmed).
To detect circumferential changes in cardiac function, we assessed twenty C57BL/6J mice at different time points after injection with LPS (10 mg/kg). Firstly, echocardiography was performed on all mice before injection. Secondly, all mice were divided into two groups for echocardiography at 6-h (n=10) and 20-h (n=10) after injection. After that, strain measurements with STE were performed.
Conventional echocardiography
Echocardiography was performed using a Vivid 7 ultrasound machine (Vivid7, GE Medical Systems, Milwaukee, Wisconsin) equipped an il3L linear probe operated at 14 MHz. Mice were imaged under light sedation (1% isoflurane in oxygen) at a room temperature of 22 °C and with decreased ambient lighting while maintained in a supine left decubitus position by an experienced handler. All mice underwent at least one echocardiography measurement to acclimate to the procedure. Echocardiographic measurements were obtained from grayscale M-mode images at the mid-papillary level in the parasternal short-axis view and 2-D mode images acquired in the parasternal long- and short-axis views. Conventional measurements of the LV included: left ventricular internal diameter at diastole (LVIDd), left ventricular internal diameter at systole (LVIDs), left ventricular volume at diastole (LVVd), left ventricular volume at systole (LVVs), EF, FS.
Speckle tracking echocardiography (STE)
Based on Lagrangian and Eulerian strain tensors of finite deformation theory, extensional strain of soft tissue in a pre-specified direction can be defined as the change in length of a segment divided by its original length [(L1−L0)/L0] (15). During each cardiac cycle, the LV undergoes a complex functional pattern of tissue deformation in multiple planes. Myocardial shortening in the circumferential axis during systole followed by reverse changes during diastole can be observed in myocardial deformation. Speckle-tracking-based strain analysis of myocardial motion (in the short-axis images) integrates frame-to-frame data from cine loops, allowing for measurements of segmental and global myocardial strain in the circumferential axes. These measures are plotted as curvilinear data for each region tracked. Similar to conventional echocardiographic measures, speckle-tracking analyses were performed offline. The data were analyzed using the following parameters: circumferential and radial strain. The first step of 2D STE image processing was to identify the ROI—the myocardium—by manual contouring of the area between endocardial and epicardial borders. Subsequently, grayscale images were analyzed using speckle tracking software following frame-to-frame movement of stable patterns of natural acoustic markers, or speckles, present in ultrasound tissue images over the cardiac cycle. Finally, the values of strain were derived from 2D STE.
Biochemical analysis
Serum cardiac Troponin-T (cTnT) was determined using Roche CARDIAC Troponin T Quantitative test from Roche Diagnostics GmbH (Mannheim, Germany), and serum CK-MB, ALT and AST were determined on a VITROS 5600 automated biochemical analyzer (Ortho-Clinical Diagnostics, New York, USA). The sample was analyzed at the central laboratory of Nanjing Medical University first affiliated hospital.
Histopathological examination
At the end of experiment, the whole heart was removed. All harvested hearts were cut into transverse blocks (2 mm thick) at the level of the papillary muscles. The tissues were immersion-fixed in 4% buffered paraformaldehyde and embedded in paraffin. Serial sections of 4 µm were cut and subjected to Haematoxylin and Eosin staining (H&E). Histological changes in erythrocyte leakage and leucocyte infiltration into cardiac interstitium were examined under a light-microscope. Myocardial leucocytes were counted according to the methods described previously (16). Briefly, myocardial leucocytes were counted on five random fields on each slide with a magnification of 200×. The individual who analyzed the histologic samples was blinded to the treatment. The infiltration of myocardial leucocytes was expressed as the average number of leucocytes per field (n=8-10/group).
Statistical analysis
Continuous variables are expressed as means ± standard deviation. Comparisons between the groups were performed using the one-way ANOVA test. Simple linear regression analyses and correlation analyses were performed to examine the relationships between strain parameters and body temperature of mice after LPS administration. Survival curves were compared by the log-rank test. All statistical tests were two-sided, and P<0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software (Version 16.0, SPSS Inc., Chicago, Illinois).
Results
Conventional echocardiography
At 6 h following injection, the myocardial volumes at systole (LVIDs, LVVs) increased while global LV function decreased significantly compared with the pre-treatment baseline values in EF (77.25%±2.96% to 58.45%±7.94%, P<0.01) and FS (40.05%±2.83% to 26.55%±4.78%, P<0.01). Decreased LVIDs (2.35±0.34 to 1.73±0.43 mm, P<0.05) and LVVs (37.20±13.37 to 16.70±12.25 µL, P<0.05) were observed in the 20-h post-LPS group along with growing global LV function evaluated by EF (71.44%±12.03%) and FS (36.08%±9.18%) compared with 6-h post-LPS group (Table 1, Figure 1A).

Full table
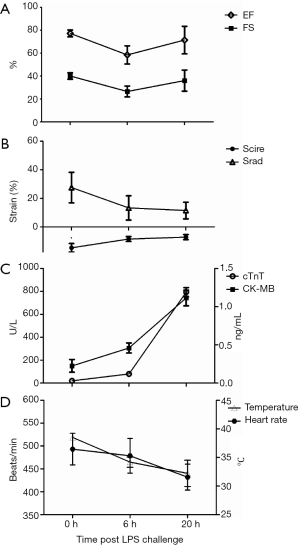
Detection of impaired circumferential changes of intrinsic myocardial function following LPS-injection
To determine the relative sensitivity of speckle-tracking based strain measures vs. conventional echocardiographic measures in the assessment of impaired cardiac function, adult mice were serially imaged following LPS injection at different time points. Advanced measures of cardiac performance (Table 1), represented by myocardial circumferential strain (−14.65%±3.00% to −8.48%±1.72%, P=0.006) and radial strain (27.55%±10.67% to 13.28%±8.49%, P=0.22), were reduced at 6 h following LPS-injection and these changes persisted over the 20-h observation period assessed by circumferential strain (−7.16%±1.79%) and radial strain (11.47%±5.82%). Both conventional and speckle-tracking based strain measures of myocardial performance exhibited similar impaired cardiac function of mice at 6 h following LPS injection, while the different results between conventional and novel measures were observed at 20-h group (Figure 1B). These differing results between conventional echocardiography and strain echocardiography warranted validation with serum and pathological evidences.
Cardiac Tropinin-T and CK-MB are released from damaged cardiac myocytes and indicate myocardial damage (17). Assessment of cTnT and CK-MB levels in serum was shown in Figure 1C, the present data revealed that the levels of cTnT and CK-MB in serum were significantly increased in the 20-h post-LPS group compared with 6-h post-LPS group (P<0.01). We also found that the 20 h-post-LPS group exhibited increased AST level (212.12±25.28 to 280.72±20.02 U/L, P<0.05), while there was no difference in ALT levels of two groups (61.44±21.88 to 61.24±20.94 U/L, P>0.05). In addition, the reduced body temperature and heart rate over time in septic mice, which indicated a decline in LV afterload, may explain why conventional echocardiography hide heart failure in severe sepsis (Figure 1D) (18,19). Finally, with deteriorated cardiac function (Figure 2). The 20-h-post-LPS group had significantly increasing leukocyte infiltration into the cardiac interstitium (Figure 3).
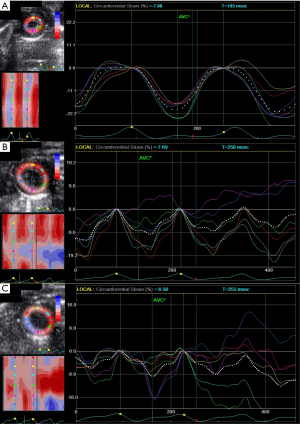
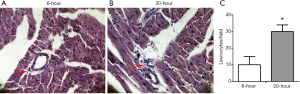
Discussion
In the present study, we investigate the role of strain imaging in the evaluation of sepsis-induced myocardial dysfunction in a mouse model. Our study demonstrates that circumferential strain from STE was able to detect impaired circumferential changes of intrinsic myocardial function after LPS-injection.
Myocardial dysfunction is common in severe sepsis but its underlying pathophysiology is not completely understood. Numerous experimental models have demonstrated that septic shock associated with reduced cardiac output and elevated systemic vascular resistance caused lethality (5,20). In recent studies, predominantly using echocardiography, myocardial dysfunction during sepsis and septic shock has been confirmed (21-23). However, most in vivo studies evaluating the effects of LPS on the heart have relied on load-dependent indexes of cardiac function, including the determination of LV EF or FS by conventional echocardiography (5,9). Since LPS may alter not only myocardial contractility, but also cardiac loading conditions, the use of such indexes may be insufficient to assess the direct effects of LPS on the intrinsic performance of the myocardium (24). Furthermore, load-dependent indexes from conventional echocardiography may even hide impaired cardiac dysfunction, because of unstable hemodynamic conditions (8,9,25,26). In our study, we observed that two load-dependent indexes (EF and FS) decreased in mice 6 h after LPS administration compared with baseline condition. Interestingly, both EF and FS resolved 20 h following LPS administration. This result was a contradiction between seemly restored normal global LV function and theoretically endotoxin-induced permanent myocardial damage. In addition, there was no gold standard for evaluating cardiac function in severe sepsis. Therefore, the measurements of serum marker and leukocyte infiltration were performed to determine cardiac inotropism. There were several evidences that exhibited impaired cardiac inotropism in LPS-induced cardiac dysfunction. First, cTnT and CK-MB served as indicators of damaged cardiac myocytes. Recent research demonstrated that high concentrations of cTnT correlated with LV dysfunction in severe sepsis and septic shock (27). Therefore, persistent elevated levels of cTnT and CK-MB showed augment myocardial injury with prolongation of LPS processing time. Second, serum AST could be released from cardiac myocytes or liver cells and increased ALT levels would mainly indicate liver injury. However, cardiac dysfunction and liver injury usually coexisted in severe sepsis. Therefore, significant elevation of plasma AST levels and no significantly changed concentration of ALT 20 h compared with 6 h following LPS treatment indicated significant myocardial injury in sepsis. Third, it was generally accepted that inflammatory mediators activated numerous molecular signals to produce cardiosupressive cytokines, resulting in septic cardiomyopathy (28). As a result, increased leukocyte infiltration leaded to the release of more inflammatory mediators 20 h than 6 h after LPS administration. Taken together, serum and histological results confirmed that myocardial injury persisted within 20 h after LPS administration in the mouse model. Consequently, load-dependent indexes (EF and FS) from conventional echocardiography masked cardiac dysfunction from LPS-induced myocardial injury.
Early pioneering research sought to distinguish between two distinct clinical profiles of septic shock (“warm shock” and “cold shock”). Based on these studies, it was concluded that patients with septic shock initially encountered an early hyper-dynamic phase from which they either recovered or fell into a hypo-dynamic phase associated with myocardial depression, heart failure, and death (4). However, there was no hyper-dynamic phase in mouse models with severe sepsis because mice were relatively endotoxin resistant, whereas humans typically showed an enhanced response (29). In our study, we observed decreased EF and FS at 6 h after LPS treatment and seemingly restored EF and FS at 20 h after LPS treatment. It has long been denied that a cardiac involvement can be a part of the septic multiple organ dysfunction syndrome since cardiac output in septic patients are usually seemingly normal or may even be elevated in comparison to the physiological range. Jianhui’s group revealed that there were significantly altered loading conditions in LPS-induced cardiac dysfunction with mouse models (24). Our study revealed that LPS administration resulted in hypothermia and decreased heart rate, which indicated significant reduction of afterload condition. Panayiotou et al. found that LPS challenged mice exhibited a marked fall in mean arterial blood pressure after 16 h which also indicated reduced afterload in mice with sepsis (30). Consequently, unstable hemodynamic conditions, including dramatic changes of body temperature, heart rate and blood pressure, are common in severe sepsis. Others have demonstrated a decline in EF of mice with increased afterload (31). Altogether, significant hemodynamic changes reduced the accuracy of conventional echocardiography in cases of severe sepsis. It was consistent with our experiment data that persistent reduced afterload enhanced EF and FS 20 h following LPS-induced septic shock.
During each cardiac cycle, the LV undergoes a complex functional pattern of tissue deformation in multiple planes. The complex interplay between these heart muscle sections produces longitudinal and circumferential shortening plus radial thickening in cardiac systole. In accordance with myocardial fiber orientation at varying levels of the LV wall, longitudinal strain is most representative of myocardial shortening at the level of the endocardium, while radial and circumferential strain values are more reflective of shortening at the level of the mid-myocardium (32). Recently, Hestenes et al. have found that LV longitudinal strain was significantly declined due to reduced afterload while LV EF remained unaltered in Escherichia coli-induced severe sepsis with pig models (33). Similarly, we have found that circumferential and radial strain declined 20 h after LPS-induced septic shock. Reduced strain was consistent with myocardial damage as indicated by serum and histological analyses, contrary to EF analysis by conventional echocardiography. Bloechlinger et al. reported that LV torsion assessed by strain echocardiography was impaired in septic shock patients (13). Therefore, strain echocardiography has significant diagnostic value under unstable hemodynamic conditions resulting from severe sepsis.
Limitation
This study utilizes a mouse model that has been treated intraperitoneally with LPS. Mouse models are most frequently used at the beginning of preclinical studies. However, they are quite resistant to endotoxin, have high heart rates, distinct hemodynamic changes, and limited blood volume in comparison with humans (34). Additionally, LPS-induced endotoxemia represents models without an infectious origin (35). However, they reflect the sepsis-induced immune response that characterizes human sepsis. Therefore, the results from experiment models with an infectious origin or clinical studies will be critical for improving clinical diagnosis and treatment.
It should be stated that inhaled isoflurane have been shown to reduce LV function and afterload (36). However, it is likely that observed data were related to LPS-induced sepsis because the inhaled concentration of isoflurane was consistent for every animal used in this study.
STE is a novel technology with numerous analysis indexes and includes multiple motion indicators. Many indicators from strain echocardiography were not considered in the present study due to small size heart of mice. Recent studies demonstrated longitudinal strain and torsion were most sensitive in detecting septic cardiac dysfunction compared with conventional echocardiographic parameters (13,14). Furthermore, we showed that circumferential strain could reliably detect cardiac dysfunction in mice with severe sepsis. Therefore, we will establish a establishing a standard evaluation system based on speckle tracking imaging for evaluating septic cardiac dysfunction in our later clinical trials.
Conclusions
This study suggests circumferential strain from STE to be a more reliable and accurate parameter in evaluating sepsis-induced cardiac dysfunction in animal model with septic cardiomyopathy, compared with conventional echocardiographic parameters. More studies are being carried out to show whether other indexes by STE exhibit improving diagnostic role of sepsis-induced myocardial depression. Therefore, our study highlights the specific and reliable role of STE for detecting cardiac contractile dysfunction during severe sepsis and septic shock.
Acknowledgements
We thank Wendy Lui, PHD in Departments of Genetics, Pediatrics and Medicine (Cardiology), Albert Einstein College of Medicine of Yeshiva University, for her critical revising of the manuscript for English language and grammar.
Funding: This work was supported by the National Natural Science Foundation of China (NSFC 81000618) to Dr. Yao Jing, (NSFC 81271589) to Prof. Xu Di and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Fernandes CJ Jr, Akamine N, Knobel E. Cardiac troponin: a new serum marker of myocardial injury in sepsis. Intensive Care Med 1999;25:1165-8. [PubMed]
- Blanco J, Muriel-Bombín A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care 2008;12:R158. [PubMed]
- Clowes GH Jr, Vucinic M, Weidner MG. Circulatory and metabolic alterations associated with survival or death in peritonitis: clinical analysis of 25 cases. Ann Surg 1966;163:866-85. [PubMed]
- MacLean LD, Mulligan WG, McLean AP, et al. Patterns of septic shock in man--a detailed study of 56 patients. Ann Surg 1967;166:543-62. [PubMed]
- Zanotti-Cavazzoni SL, Goldfarb RD. Animal models of sepsis. Crit Care Clin 2009;25:703-19. vii-viii. [PubMed]
- Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med 2009;37:S30-7. [PubMed]
- Fink MP. Animal models of sepsis. Virulence 2014;5:143-53. [PubMed]
- Parker MM, Shelhamer JH, Bacharach SL, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 1984;100:483-90. [PubMed]
- Peters MJ, Brierley J. No representation without taxation: declaration of (load) independence in septic cardiomyopathy. Pediatr Crit Care Med 2012;13:349-50. [PubMed]
- Werdan K, Oelke A, Hettwer S, et al. Septic cardiomyopathy: hemodynamic quantification, occurrence, and prognostic implications. Clin Res Cardiol 2011;100:661-8. [PubMed]
- Huang SJ, Orde S. From speckle tracking echocardiography to torsion: research tool today, clinical practice tomorrow. Curr Opin Crit Care 2013;19:250-7. [PubMed]
- Tee M, Noble JA, Bluemke DA. Imaging techniques for cardiac strain and deformation: comparison of echocardiography, cardiac magnetic resonance and cardiac computed tomography. Expert Rev Cardiovasc Ther 2013;11:221-31. [PubMed]
- Bloechlinger S, Berger D, Bryner J, et al. Left ventricular torsion abnormalities in septic shock and corrective effect of volume loading: a pilot study. Can J Cardiol 2013;29:1665-71. [PubMed]
- Basu S, Frank LH, Fenton KE, et al. Two-dimensional speckle tracking imaging detects impaired myocardial performance in children with septic shock, not recognized by conventional echocardiography. Pediatr Crit Care Med 2012;13:259-64. [PubMed]
- Heimdal A, Støylen A, Torp H, et al. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr 1998;11:1013-9. [PubMed]
- Sennoun N, Meziani F, Dessebe O, et al. Activated protein C improves lipopolysaccharide-induced cardiovascular dysfunction by decreasing tissular inflammation and oxidative stress. Crit Care Med 2009;37:246-55. [PubMed]
- Røsjø H, Varpula M, Hagve TA, et al. Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med 2011;37:77-85. [PubMed]
- Hoffman-Goetz L, Keir R. Fever and survival in aged mice after endotoxin challenge. J Gerontol 1985;40:15-22. [PubMed]
- Saito H, Sherwood ER, Varma TK, et al. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev 2003;124:1047-58. [PubMed]
- Wiel E, Costecalde ME, Lebuffe G, et al. Activated protein C increases sensitivity to vasoconstriction in rabbit Escherichia coli endotoxin-induced shock. Crit Care 2006;10:R47. [PubMed]
- Jardin F, Fourme T, Page B, et al. Persistent preload defect in severe sepsis despite fluid loading: A longitudinal echocardiographic study in patients with septic shock. Chest 1999;116:1354-9. [PubMed]
- Vincent JL, Gris P, Coffernils M, et al. Myocardial depression characterizes the fatal course of septic shock. Surgery 1992;111:660-7. [PubMed]
- Vieillard Baron A, Schmitt JM, Beauchet A, et al. Early preload adaptation in septic shock? A transesophageal echocardiographic study. Anesthesiology 2001;94:400-6. [PubMed]
- Jianhui L, Rosenblatt-Velin N, Loukili N, et al. Endotoxin impairs cardiac hemodynamics by affecting loading conditions but not by reducing cardiac inotropism. Am J Physiol Heart Circ Physiol 2010;299:H492-501. [PubMed]
- Zanotti Cavazzoni SL, Guglielmi M, Parrillo JE, et al. Ventricular dilation is associated with improved cardiovascular performance and survival in sepsis. Chest 2010;138:848-55. [PubMed]
- Parrillo JE. The cardiovascular pathophysiology of sepsis. Annu Rev Med 1989;40:469-85. [PubMed]
- Landesberg G, Jaffe AS, Gilon D, et al. Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation*. Crit Care Med 2014;42:790-800. [PubMed]
- Flierl MA, Rittirsch D, Huber-Lang MS, et al. Molecular events in the cardiomyopathy of sepsis. Mol Med 2008;14:327-36. [PubMed]
- Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, et al. Experimental models of sepsis and their clinical relevance. Shock 2008;30 Suppl 1:53-9. [PubMed]
- Panayiotou CM, Baliga R, Stidwill R, et al. Resistance to endotoxic shock in mice lacking natriuretic peptide receptor-A. Br J Pharmacol 2010;160:2045-54. [PubMed]
- Van den Bergh A, Flameng W, Herijgers P. Parameters of ventricular contractility in mice: influence of load and sensitivity to changes in inotropic state. Pflugers Arch 2008;455:987-94. [PubMed]
- Ram R, Mickelsen DM, Theodoropoulos C, et al. New approaches in small animal echocardiography: imaging the sounds of silence. Am J Physiol Heart Circ Physiol 2011;301:H1765-80. [PubMed]
- Hestenes SM, Halvorsen PS, Skulstad H, et al. Advantages of strain echocardiography in assessment of myocardial function in severe sepsis: an experimental study. Crit Care Med 2014;42:e432-40. [PubMed]
- Michie HR. The value of animal models in the development of new drugs for the treatment of the sepsis syndrome. J Antimicrob Chemother 1998;41 Suppl A:47-9.
- Freise H, Brückner UB, Spiegel HU. Animal models of sepsis. J Invest Surg 2001;14:195-212. [PubMed]
- Bernard JM, Wouters PF, Doursout MF, et al. Effects of sevoflurane and isoflurane on cardiac and coronary dynamics in chronically instrumented dogs. Anesthesiology 1990;72:659-62. [PubMed]


