Hydration with magnesium and mannitol without furosemide prevents the nephrotoxicity induced by cisplatin and pemetrexed in patients with advanced non-small cell lung cancer
Abstract
Background: The aim of this study was to examine the effect of hydration with magnesium and mannitol without
furosemide on the nephrotoxocity accompanying combination chemotherapy using cisplatin and pemetrexed in patients
with advanced non-small cell lung cancer (NSCLC).
Methods: Fifty patients with NSCLC who received cisplatin plus pemetrexed, using either old hydration protocol including
normal saline with mannitol and furosemide, or a new one including normal saline with magnesium and mannitol without
furosemide were retrospectively analyzed. Nephrotoxicity was compared between patients treated using the old protocol
and those treated with the new protocol. Univariate and multivariate analyses were performed to identify the independent
factors associated with protection against nephrotoxicity in patients with NSCLC who received cisplatin plus pemetrexed.
Results: Thirty patients received the old hydration protocol, while 20 patients were treated using the new hydration
protocol. The patients treated using the new hydration protocol showed a significantly greater increase in creatinine
clearance (P=0.0004) and a decrease in the serum creatinine level (P=0.0148) after one course of chemotherapy compared
with those treated using the old hydration protocol. There were no differences in the chemotherapeutic response or overall
survival between the groups (P=0.572). The new hydration protocol with supplemented magnesium with mannitol without
furosemide was an independent factor for the protection against nephrotoxicity induced by cisplatin and pemetrexed in
patients with advanced NSCLC [HR 0.232 (95% CI: 0.055-0.986), P=0.039].
Conclusions: These results demonstrate that the new hydration protocol comprising supplementation with magnesium
without furosemide could prevent the nephrotoxicity induced by cisplatin and pemetrexed without affecting the
treatment outcome.
Key words: Lung cancer; cisplatin; magnesium; nephrotoxicity; pemetrexed
Introduction
Lung cancer is a leading cause of death in Japan and other developed countries (1). More than 60% of patients with lung cancer, especially those with non-small cell lung cancer (NSCLC), are inoperable at the time of diagnosis and need to receive chemotherapy containing cisplatin (2). Recently, the percentage of adenocarcinomas among cases of NSCLC has been increasing (3), therefore, regimens containing cisplatin and pemetrexed are expected to be more frequently used (4). Cisplatin is one of the most active and widely used drugs, and still remains a standard component of combination chemotherapy for lung cancer (5). However, nephrotoxicity is a well-known side effect of cisplatin treatment (6). The logistic regression analyses have shown that the risk factors for the development of nephrotoxicity include older age, female gender, current smoking, and hypoalbuminemia (7). The nephrotoxic damage appears to be a clinical problem in 28-42% of patients who receive cisplatin (8). Therefore, many researchers have sought less toxic methods for administering cisplatin. It has been reported that hydration with magnesium supplementation can reduce the nephrotoxocity induced by cisplatin (8,9). In contrast, the protective effect of furosemide against nephrotoxicity is still controversial (10). In fact, Lehane et al. reported that high doses of furosemide cause nephrotoxicity, and it has been suggested that its use with cisplatin may aggravate the nephrotoxicity (11). To the best of our knowledge, there have been no studies that have examined the protective effect of hydration using saline lacking furosemide supplemented with magnesium and mannitol on the nephrotoxicity induced by cisplatin and pemetrexed in patients with advanced non-squamous NSCLC.
We recently established a new hydration method using 2,700 mL of saline supplemented with magnesium and mannitol without furosemide, and examined the effect of this hydration protocol on the protection against the nephrotoxicity induced by cisplatin and pemetrexed in non-squamous NSCLC patients.
Subjects and methods
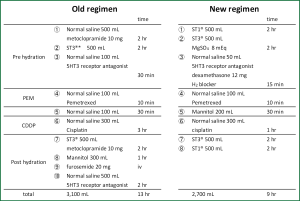
Fifty patients with NSCLC who received cisplatin (75 mg/m2) plus pemetrexed (500 mg/m2) from May 2009 to March 2012 were retrospectively analyzed in this study. Chemotherapy was repeated every three weeks unless otherwise noted. Until July 2010, the old hydration protocol, comprising 3,100 mL of normal saline with mannitol (300 mL) and furosemide (20 mg), was used for 30 patients (old group). Because of the relatively high incidence of renal toxicity for old hydration group, the old hydration protocol was replaced with a new protocol, which contained 2,700 mL of normal saline lacking furosemide with mannitol (200 mL) and magnesium sulfate (8 mEq), and was given to 20 patients (new group). Both regimens are shown in Figure 1. Nephrotoxicity was evaluated by the both serum creatinine level and creatinine clearance (Ccr) and were compared between patients treated with the old and new protocols. The serum creatinine level was measured by an enzymatic method. The Ccr was calculated with Cockcroft & Gault’s formula (12). Hematological toxicities and non-hematological toxicities except renal toxicity were defined according to the Common Terminology Criteria for Adverse Event (CTCAE) version 4.0. The efficacy of chemotherapy was evaluated based on the response rate, disease control rate and overall survival according to the RECIST criteria, version 1.1. Comprehensive informed consent was obtained from all patients.
Statistical analyses
To evaluate the differences in the patients’ characteristics, toxicities, and chemotherapeutic responses, the chi-square test was used. The differences in the nephrotoxicity (evidenced by the ⊿serum creatinine and ⊿Ccr) between the old regimen and new regimen were analyzed with Mann-Whitney’s U test. To analyze the overall survival (OS), survival curves were drawn by the Kaplan-Meier method. The OS was calculated from the date of initiation of chemotherapy to the date of death. The OS rates were compared using the log-rank test according to the regimen (old regimen vs. new regimen). The univariate and multivariate analyses using a multiple regression analysis were performed to identify the independent factors associated with protection against nephrotoxicity in patients with non-squamous NSCLC who received cisplatin plus pemetrexed using the SPSS (IBM, USA, New York), software program, version 19. P values <0.05 were considered to be statistically significant.
Results
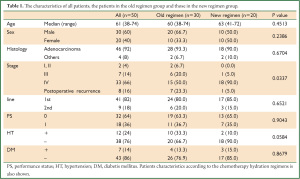
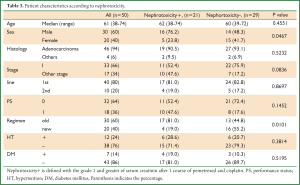
The characteristics of all patients, the patients in the old regimen group and those in the new regimen group are shown in Table 1. The median age of all patients was 61 years old (range, 38-74 years old). Of the total of 50 patients, 30 patients were males (60%), 47 patients had adenocarcinoma (94%). The clinical stage was I and II in two, III in seven, IV in 33 and postoperative recurrence in eight patients. The ECOG performance status was 0 in 32 patients and 1 in 18 patients. As a co-morbidity related to the renal toxicity, 12 patients had hypertension (HT) and seven had diabetes mellitus (DM). There were no significant differences in the patient age, gender, performance status, histology, administered lines of treatment or incidence of DM between the groups. With regard to HT, patients in the new regimen group tended to less frequently have HT than those in the old group. However, there were no significant differences between the groups. In contrast, the old regimen group included more advanced stage patients compared to the new regimen group.
Full table
The completion rate of four cycles was 43.5% and 46.7% for the old and new regimen groups, respectively (data not shown). This difference was not statistically significant. The change in the serum creatinine level (maximum serum creatinine during one course of chemotherapy - serum creatinine before chemotherapy) and the change in the Ccr (Ccr before chemotherapy - nadir Ccr during one course of chemotherapy) were calculated. Figure 2A and B show the comparison of the change in the serum creatinine and the change in the Ccr in both groups. The nephrotoxicity in the old regimen group was more severe than that in the new regimen group.

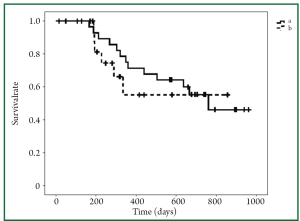
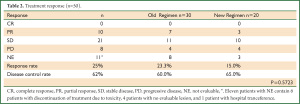
We also compared other toxicities, besides renal dysfunction, between the groups. Both groups demonstrated similar toxicity profiles, and there were no significant differences in any of the other toxicities between the groups (data not shown). Of note, the response rate and disease control rate in the two groups were also not significantly different (Table 2). Even though the overall survival in the new regimen group did not reach the median survival time, the overall survival in the new regimen group appears to be equivalent to that in the old regimen group (Figure 3).
Full table
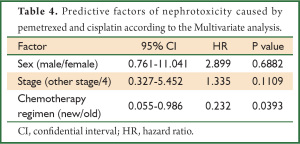
We next performed a univariate analysis to identify the factors influencing renal toxicity. As shown in Table 3, renal toxicity was more frequently observed in males and in the old regimen group than in females and in the new regimen group. Interestingly, there were no differences in nephrotoxicity based on the patient age, histology, stage, performance status, or co-morbidities (HT and DM) in the two groups. According to the multivariate analysis (Table 4), only the new chemotherapy regimen was an independent factor predicting the protection of renal toxicity caused by cisplatin and pemetrexed.
Full table
Full table
Discussion
In this study, we clearly demonstrated that a new regimen utilizing 2,700 mL of saline lacking furosemide supplemented with magnesium and mannitol with rapid cisplatin infusion (300 mL of saline containing 75 mg/m2 cisplatin in 1 hour) would be useful to avoid the renal toxicity caused by cisplatin and pemetrexed, without any reduction in the efficacy of the regimen in patients with advanced non-squamous NSCLC. To exclude the possibility that the patient background, such as age, gender, stage and co-morbidities, would affect the renal toxicity induced by cisplatin and pemetrexed, a multivariate analysis using a multiple regression method was performed, and revealed that the new regimen was an independent predictive factor for the protection against the nephrotoxicity induced by cisplatin and pemetrexed.
There has been speculation about what ingredients (factors) in the new regimen contributed to protecting against the nephrotoxicity induced by cisplatin and pemetrexed. Several researchers had previously reported that magnesium supplementation protected against cisplatin-induced nephrotoxicity (8,9). Willox et al. performed a randomized trial to evaluate the effect of magnesium supplementation in testicular cancer patients receiving cisplatin, and demonstrated its effect on renal protection (9). Bodnar et al. have revealed the nephroprotective effect of magnesium supplementation during chemotherapy with cisplatin in patients with epithelial ovarian cancer (8). In addition, Lajer et al. reported that magnesium depletion enhances cisplatin-induced nephrotoxicity (13). Based on these previous reports, the magnesium supplementation in the new regimen appears to have been at least partly responsible for the reduced incidence of nephrotoxicity in this study.
Mannitol causes osmotic diuresis. Hayes and Frick revealed that mannitol decreased cisplatin nephrotoxicity (14,15). Clinically, mannitol reduces the urine concentration of cisplatin, and this effect is considered to be the mechanism underlying the amelioration of renal toxicity. Since most of the previous reports supported its effect on nephroprotection, except one controversial paper in which hydration with saline + mannitol was not nephroprotective compared to saline alone, mannitol was included in our new regimen (16).
Although other researchers have already reported the effect of furosemide on reducing the renal toxicity, its effect on the prevention of nephrotoxicity is still controversial (10). In fact, it has been reported that furosemide protects renal function, while it worsens renal histopathology (11). Moreover, McMurtry et al. reported that furosemide enhances rodent nephrotoxicity (11). Therefore, furosemide was not included in the new regimen, resulting in amelioration of the nephrotoxicity induced by cisplatin and pemetrexed. We also employed the rapid infusion of cisplatin (75 mg/m2/300 mL/1 hour) in the new regimen. The nephrotoxicity induced by cisplatin is related to the contact time of free cisplatin to the proximal tubules in the kidneys (17). Therefore, the rapid cisplatin infusion method used in the new regimen might have contributed to the reduction of cisplatin-induced nephrotoxicity. The new regimen involving the supplementation with magnesium and mannitol, in concert with rapid cisplatin infusion, could prevent cisplatin-induced nephrotoxicity.
It has been reported that supplementation with magnesium would affect in vitro and in vivo tumor growth (18). In fact, Parsons et al. have suggested that magnesium depletion decreases tumor growth (19). However, there were no statistically significant differences in the chemotherapeutic response or overall survival between the groups in our study, although the clinical outcomes appeared to be a little bit better in patients treated using the old regimen compared to those treated with the new regimen. Our results are supported by the report by Willox et al., in which the supplementation with magnesium did not modify the chemotherapeutic response or prognosis for patients with testicular cancer (9).
There are several limitations in this study, which need to be addressed. Our study population was relatively small and retrospective, large scale and prospective studies are needed to confirm the utility of this protocol. In addition, there are several differences between the old regimen and the new regimen besides magnesium and furosemide. For instance, dexamethasone is contained in the new regimen, while it is not contained in the old regimen. We could not exclude the possibility that addition of dexamethanose in the new regimen was useful to prevent cisplatin-induced nehrotoxicity.
In conclusion, we clearly demonstrated that saline hydration without furosemide, supplemented with magnesium and mannitol, with rapid cisplatin infusion ameliorated the nephrotoxicity caused by cisplatin and pemetrexed in patients with advanced non-squamous NSCLC.
Acknowledgements
We thank all of the collaborators involved in this study in our department at Jutendo University.
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220-41.
- van Meerbeeck JP. Staging of non-small cell lung cancer: consensus, controversies and challenges. Lung Cancer 2001;34:S95-107.
- Yano T, Haro A, Shikada Y, et al. Non-small cell lung cancer in never smokers as a representative 'non-smoking-associated lung cancer': epidemiology and clinical features. Int J Clin Oncol 2011;16:287-93.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51.
- Johnson SW, O’Dwyer PJ. Pharmacology of cancer chemotherapy: cisplatin and its analogues. In: De Vita VT Jr, Hellman S, Rosenberg SA. Eds. Cancer, Principles and practice of oncology, 7th ed. Philadelphia: Lippincott Williams & Wilkins, 2005:344-58.
- dos Santos NA, Carvalho Rodrigues MA, Martins NM, et al. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol 2012;86:1233-50.
- Lebwohl D, Canetta R. Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer 1998;34:1522-34.
- Bodnar L, Wcislo G, Gasowska-Bodnar A, et al. Renal protection with magnesium subcarbonate and magnesium sulphate in patients with epithelial ovarian cancer after cisplatin and paclitaxel chemotherapy: a randomised phase II study. Eur J Cancer 2008;44:2608-14.
- Willox JC, McAllister EJ, Sangster G, et al. Effects of magnesium supplementation in testicular cancer patients receiving cis-platin: a randomised trial. Br J Cancer 1986;54:19-23.
- Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol 1993;50:147-58.
- Lehane D, Winston A, Gray R, et al. The effect of diuretic pre-treatment on clinical, morphological and ultrastructural cis-platinum induced nephrotoxicity. Int J Radiat Oncol Biol Phys 1979;5:1393-9.
- Haim N, Oman SD, Galai N, et al. Estimation of creatinine clearance without 24-hour urine collection--a useful guide during cisplatin therapy. Acta Oncol 1993;32:409-12.
- Lajer H, Kristensen M, Hansen HH, et al. Magnesium depletion enhances cisplatin-induced nephrotoxicity. Cancer Chemother Pharmacol 2005;56:535-42.
- Hayes DM, Cvitkovic E, Golbey RB, et al. High dose cis-platinum diammine dichloride: amelioration of renal toxicity by mannitol diuresis. Cancer 1977;39:1372-81.
- Frick GA, Ballentine R, Driever CW, et al. Renal excretion kinetics of high-dose cis-dichlorodiammineplatinum(II) administered with hydration and mannitol diuresis. Cancer Treat Rep 1979;63:13-6.
- Santoso JT, Lucci JA 3rd, Coleman RL, et al. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: a randomized trial. Cancer Chemother Pharmacol 2003;52:13-8.
- Tiseo M, Martelli O, Mancuso A, et al. Short hydration regimen and nephrotoxicity of intermediate to high-dose cisplatin-based chemotherapy for outpatient treatment in lung cancer and mesothelioma. Tumori 2007;93:138-44.
- Wolf FI, Cittadini AR, Maier JA. Magnesium and tumors: ally or foe? Cancer Treat Rev 2009;35:378-82.
- Parsons FM, Edwards GF, Anderson CK, et al. Regression of malignant tumours in magnesium and potassium depletion induced by diet and haemodialysis. Lancet 1974;1:243-4.


