Pneumothorax, an underappreciated complication with an airway exchange catheter
Abstract
While airway exchange catheters (AEC) are designed to safely maintain a secure airway and sometimes allow for ventilation while exchanging an endotracheal tube or performing a trial tracheal extubation, their use is sometimes associated with devastating complications. Pneumothorax is an underappreciated complication with AECs that can occur even in the absence of high pressure ventilation with quick clinical deterioration. The development of a pneumothorax can be difficult to distinguish from other potential causes of clinical deterioration and the clinician should maintain a high level of suspicion for quick diagnosis and treatment. Here we report a case of tension pneumothorax leading to cardiovascular collapse that occurred very suddenly with the introduction of an AEC. This pneumothorax presented in an atypical manner by all monitors available except for blood pressure monitoring. Therefore this case highlights the need for strong clinical suspicion of pneumothorax with the use of AECs.
Key words: Airway exchange catheters (AEC); pneumothorax
Introduction
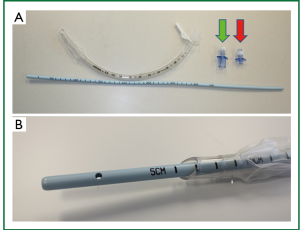
Airway exchange catheters (AECs) are widely available products that provide a conduit within the trachea that allows for emergent securing or re-securing of an airway and, in some cases, ventilation during extubation or exchange of an airway device (Figure 1). Organizations, such as the Difficult Airway Society (the second-largest anesthesia specialty organization in Europe) endorse the use of the AEC in extubation of the “at risk” airway. Therefore AECs are commonly used in those situations, although their exact place in the management of a difficult airway remains unclear and its use is currently up to the judgment of the individual practitioner (1).
We present a case of tension pneumothorax in an anesthetized patient with the use of an AEC whose clinical presentation made for timely diagnosis very challenging. Definitive management depended on the identification and treatment of tension pneumothorax.
Case report
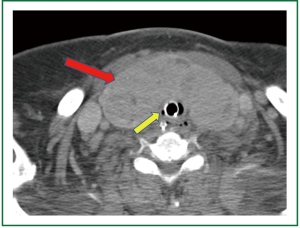
A 74 year old woman was referred for tracheostomy and open gastrostomy tube placement 17 days after suffering a hemorrhagic cerebrovascular accident secondary to a ruptured cerebral aneurysm. At baseline this patient’s Glasgow Coma Score was rated at 4 and the above procedure was scheduled in preparation for transfer to a skilled nursing facility. The patient’s medical history was significant for diastolic congestive heart failure, coronary artery disease, hypertension, aortic and tricuspid valve disease, mitral valve repair, morbid obesity, possible untreated chronic obstructive pulmonary disease, and a large thyroid mass. The thyroid mass was predominantly midline and completely covering the anterior and lateral surfaces of the trachea including the proposed surgical location for tracheostomy insertion (Figure 2). Therefore plans were made for a resection of the thyroid isthmus with central thyroidectomy for proper exposure prior to tracheostomy.
Given concern for protection of the airway through a potentially difficult surgical field in a patient not likely to tolerate long periods of apnea, the anesthetic plan included passing a Cook Medical Aintree AEC (Figure 1), a hollow AEC 4.7 mm in diameter with a blunt tip and adapter for connection to a jet ventilator, for airway protection while the oral endotracheal tube (ETT) was exchanged for a tracheostomy airway device. The primary advantage of this anesthetic plan was to maintain the patient’s airway in the event of an obstructed surgical field and interval ventilation through the device itself should the procedure prove long and technically difficult.
The patient was transported to the operating room and anesthesia induced gently with a low concentration of inhaled anesthetic and intravenous paralytic, and maintained in the same fashion along with small doses of narcotics as necessary. The gastrostomy was easily performed first then the surgical teams proceeded with the thyroid isthmusectomy portion of the procedure without obvious complications, only noting continuous oozing of blood from the margins of the remaining thyroid tissue.
The AEC was then carefully passed down the ETT noting the depth along the markings of the AEC in correlation with the ETT and attached to a jet ventilator. Manual jet ventilation was then begun; however, the patient immediately experienced significant hypotension as noted by the arterial line blood pressure tracing, with no more than two breaths having been delivered by jet ventilation. The ETT was replaced to its original position and the AEC was immediately removed and ventilation resumed through the ETT. Of note, saturation of peripheral oxygen by standard pulse oxymetry remained 98-100%, heart rate did not increase above 20% of baseline, peak and plateau airway pressures and tidal volumes were noted to be nearly identical on resumption of ventilation through the ETT and end tidal CO2 values decreased less than 10% of previous values. Standard five lead electrocardiogram leads were in place and intraoperative ECG showed no significant changes from baseline.
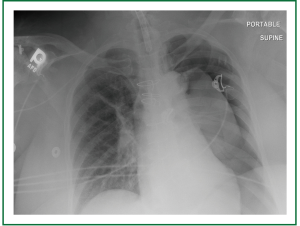
Supportive measures were immediately undertaken with multiple intravenous vasoactive medications, and blood pressure ultimately responded only to escalating doses of epinephrine and an epinephrine infusion. Given the above measurements and patient’s medical history, a cardiac event was considered most likely, but a jet ventilator associated complication could not be ruled out so no repeated attempts were made at jet ventilation. Due to the fact that the trachea was at this time fully exposed, the decision was made to insert the tracheostomy. Incision was made in the trachea and trachesotomy was inserted without incident. Upon arrival to the ICU, the patient continued to have hypotension. Decreased breath sounds were suspected on the left. A chest x-ray shot immediately after this finding confirmed large left-sided tension pneumothorax with mediastinal shift and evidence of left heart compression (Figure 3). A chest tube was introduced immediately relieving a large volume of air and allowing the weaning of the epinephrine infusion, with complete liberation of the infusion by post-operative day two. The patient was eventually deemed medically stable for transfer to a skilled nursing facility, after prolonged hospitalization, with a functional tracheostomy airway and nutrition with gastrostomy tube feeds.
Discussion
When an airway is deemed to be difficult, AECs are often employed as part of the tracheal extubation strategy to facilitate potential re-intubation. Many types of AECs exist that are designed to protect a tenuous airway and some even allow for ventilation through the AEC itself, as in the case of the Aintree AEC described here. An AEC can be passed into the lumen of an in situ ETT or directly into the patient’s airway with greater ease as these products are thinner and longer than a standard ETT (Figure 1). When extubated with an AEC in place, a patient can be monitored for signs of deterioration and re-intubation of the trachea can be accomplished over the AEC quickly, even in the case of a difficult airway. Furthermore, some AECs allow for ventilation through the lumen of the device itself, providing another method to maintain oxygenation in critical situations. Recent concerns for safety with these devices, however, have called into question when they should be used and preparations that should be made for their potential complications (2,3).
The overall concept of these devices is that of a long, thin, flexible, sometimes hollow tube with a blunt end that can accomplish the above objectives with minimal dangers to the patient. Ample literature exists, however, that proves these products can have a high rate of very dangerous complications as high as 60% (4-6). The most ominous of these complications is pneumothorax, which is up to 11% (3,7). While pneumothorax is a known complication of high pressure jet ventilation (HPJV) as a result of barotrauma, auto-PEEP, and dynamic hyperinflation (6,8-11), pneumothorax has been reported with the use of AECs without HPJV (2,3).
Thus, two mechanisms are suggested for the development of a pneumothorax with AEC use. The first mechanism is trauma to the tracheobronchial tree by the device itself. In spite of their blunt tips, AECs have been reported to cause tears to the tracheobronchial tree from the catheter tip and misdirection of the catheter tip through the Murphy Eye of the ETT that can quickly lead to a fatal pneumothorax, the risk of which is increased with smaller AECs (3). The second mechanism is barotrauma associated with jet ventilation. High Frequency Jet Ventilation (HFJV) is known to cause pneumothorax through dynamic hyperinflation and auto-peak end expiratory pressure (PEEP) leading to barotrauma and pneumothorax, and this risk is increased when an AEC is improperly placed delivering high pressures to smaller airway segments (6,8-10). Good technique is therefore essential in AEC placement and jet ventilation with a vigilant practitioner monitoring for low airway pressures, appropriate chest rise and fall, and ample time for exhalation (12).
Pneumothorax is suspected when tachycardia, tachypnea, hypertension, or oxyhemoglobin desaturation occurs along with a new unilateral reduction of breath sounds. Hypotension and tracheal deviation are suspicious of tension pneumothorax, and definitive diagnosis traditionally depends on chest radiograph and/or improvement with therapeutic intervention, i.e., decompression. These signs can be more difficult to recognize intra-operatively in the anesthetized patient with the challenges of performing imaging on the acutely decompensating patient (13). Therefore a strong clinical suspicion is in need for the safe use of an AEC in order to quickly recognize and treat pneumothorax even in the setting of vague clinical and diagnostic data.
The severity of these complications has lead to investigations into alternatives for the use of AECs and debate on the risks and benefits in selecting these devices for an anesthetic plan. When choosing an anesthetic plan, it is clear that the anesthesia provider should be aware of the risks of these devices and be vigilant for the development of complications, particularly pneumothorax even in the face of unclear data immediately available, so that treatment can be started rapidly, the trachea reintubated with a standard ETT, and the AEC removed (3,7,14,15).
Importantly, this case does demonstrate a delay in diagnosis of a potentially fatal tension pneumothorax. In the acutely decompensating patient, practitioners should remember to revert back to the “ABC’s” of trauma resuscitation. Confirmation of the airway by capnography, followed by judicious auscultation of the lung fields in the operating room may have lead to a prompt diagnosis and treatment. Attributing the decompensation to “a cardiac event” should almost be a diagnosis of exclusion until other potentially “fixable” causes are ruled out. The importance of ample communication between anesthesia provider and surgeon about the condition of the patient can also not be over-emphasized.
In summary, the use of an AEC should require careful decision making on the part of the clinician taking into account many factors including their rate of complication and the patient’s risk of loss of airway. This case represents an atypical presentation for a pneumothorax given available data at the time, and therefore clinical suspicion for pneumothorax should always remain high in the use of AECs and immediately ruled out in the event of cardiovascular or pulmonary collapse.
Acknowledgements
Kazuaki Takabe is supported by United States National Institute of Health (R01CA160688) and Susan G. Komen for the Cure (Investigator Initiated Research Grant (12222224).
Disclosure: The authors declare no conflict of interest.
References
- American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2003;98:1269-77.
- Harris K, Chalhoub M, Maroun R, et al. Endotracheal tube exchangers: should we look for safer alternatives? Heart Lung 2012;41:67-9.
- Duggan LV, Law JA, Murphy MF. Brief review: Supplementing oxygen through an airway exchange catheter: efficacy, complications, and recommendations. Can J Anaesth 2011;58:560-8.
- Benumof JL. Airway exchange catheters: simple concept, potentially great danger. Anesthesiology 1999;91:342-4.
- Baraka AS. Tension pneumothorax complicating jet ventilation via a cook airway exchange catheter. Anesthesiology 1999;91:557-8.
- Mort TC. Continuous airway access for the difficult extubation: the efficacy of the airway exchange catheter. Anesth Analg 2007;105:1357-62.
- Cooper RM. The use of an endotracheal ventilation catheter in the management of difficult extubations. Can J Anaesth 1996;43:90-3.
- Gluck E, Heard S, Patel C, et al. Use of ultrahigh frequency ventilation in patients with ARDS. A preliminary report. Chest 1993;103:1413-20.
- Nunn C, Uffman J, Bhananker SM. Bilateral tension pneumothoraces following jet ventilation via an airway exchange catheter. J Anesth 2007;21:76-9.
- Dworkin R, Benumof JL, Benumof R, et al. The effective tracheal diameter that causes air trapping during jet ventilation. J Cardiothorac Anesth 1990;4:731-6.
- Mort TC, Meisterling EM, Waberski WM. Exchanging a tracheal tube in the ICU patient: A comparison of two exchangers with direct laryngoscopy. Anesthesiology 1997;87:240 A.
- Mace SE, Khan N. Needle cricothyrotomy. Emerg Med Clin North Am 2008;26:1085-101, xi.
- Omar HR, Mangar D, Camporesi EM. Utilization of intraoperative transthoracic ultrasound for diagnosis of pneumothorax. Anesthesiology 2012;116:967-8; author reply 968-9.
- Dosemeci L, Yilmaz M, Yegin A, et al. The routine use of pediatric airway exchange catheter after extubation of adult patients who have undergone maxillofacial or major neck surgery: a clinical observational study. Crit Care 2004;8:R385-90.
- Benumof JL. Airway exchange catheters: simple concept, potentially great danger. Anesthesiology 1999;91:342-4.


