Changes in brain natriuretic peptide are correlated with changes in global end-diastolic volume index
Introduction
Brain natriuretic peptide (BNP) was first isolated from porcine brain and then was found to be synthesized by myocytes and fibroblast in the atria and ventricle in response to left ventricular filling pressure and wall stress (1). Since its discovery, BNP has been extensively studied in many clinical settings. In urgent care setting, it has been consistently proven to be a helpful tool in differentiating dyspnea caused by chronic heart failure from noncardiac dyspnea (2,3), and BNP testing has become a standard part of the evaluation in patients presented to the emergency department with dyspnea. In chronic heart failure, circulating concentrations of BNP are elevated in proportion to the severity of symptoms and the degree of ventricular dysfunction (4,5). Additionally, the measurement of a single BNP level significantly improves the management of patients with acute dyspnea, and thus reduces the length of hospital stay and medical costs (6).
Stretch of cardiomyocytes is believed to be the most important stimulus of BNP regulation (7), and many human studies have focused on the correlation between BNP levels and cardiac filling pressure (8). Although a significant correlation exists between serum BNP levels and cardiac filling pressure, this correlation is not sufficiently strong to make BNP a reliable surrogate indicator of cardiac filling pressure (9-11). However, a large body of evidence suggests that cardiac filling pressure may not be a good indicator of the stretch of cardiomyocytes, especially in the critical care setting (12,13). Factors such as mechanical ventilation, use of vasoactive agents and cardiac wall compliance all contribute significantly to the measurement of filling pressure. Thus, it is not surprising that the filling pressure is only modestly correlated to plasma BNP.
With the development of new techniques, more indices of cardiac performance can be measured with less invasive technique. PiCCO-technology is one of such system that can provide many cardiac parameters by using thermodilution method (14). Global end diastolic volume (GEDV) is an index that can be measured with this system and has been found to be a more accurate indicator of cardiac preload than filling pressures (15,16). However, the correlation between plasma BNP and GEDV has not been extensively studied. To our best knowledge, only one small study enrolled eight patients has been conducted in this area (17). Thus, to better establish the correlation between plasma BNP and GEDVI, we conducted this prospective study in a cohort of critically ill patients who required PiCCO monitoring. Because BNP concentrations are influenced by many factors such as sex, age, renal function and cardiac impairment, a single value of BNP may not be related to a fixed volume status. However, because factors influencing BNP concentrations remain unchanged in a single patient within a short period, we hypothesized that changes in BNP was correlated with changes in GEDVI and serial measurements of BNP could reflect changes in volume status in an individual patient.
Methods
Study population
The study was conducted between August 2010 and August 2011 in a tertiary teaching hospital. Patients admitted to the 18-bed intensive care unit (ICU) and required PiCCO monitoring were evaluated for inclusion. Indications for PiCCO monitoring were: (I) shock defined by the presence of 4 criteria (Heart rate of at least 90/min; a respiratory rate of at least 20/min or a PaCO2 of 32 mmHg or lower or the use of mechanical ventilation; the use of vasopressors to maintain a systolic blood pressure of at least 90 mmHg despite fluid resuscitation, low dose of dopamine (≤5 μg/kg per minute), or dobutamine; at least 1 of 3 signs of hypoperfusion (urine output <0.5 mL/kg of body weight per hour for 1 hour or more; neurologic dysfunction defined by confusion, psychosis, or a Glasgow coma scale score of ≤6; plasma lactate higher than the upper limit of the normal value); and (II) acute respiratory distress syndrome (ARDS) defined by the presence of more than 24 hours of 4 criteria: acute decrease in PaO2/FIO2 to 200 mmHg or lower, whatever the level of positive end-expiratory pressure; bilateral pulmonary infiltrates or a chest radiograph consistent with edema; no clinical evidence of left atrial hypertension; and requirement for positive pressure ventilation. Patients were excluded if they were: younger than 18 years, older than 80, moribund, experienced hemorrhagic shock, or had thrombocytopenia (≤10.0×109/L) or renal dysfunction (serum creatinine >200 μmol/L), or the patient signed do-not-resuscitation order. Baseline characteristics including sex, age, clinical diagnosis, APACHII score and reasons for hemodynamic monitoring were recorded at ICU admission. During treatment, valuables such as fluid balance, use of vasoactive agents and mechanical ventilation were recorded. Because this was an observational study, informed consent was waived. The study was approved by the Ethics Committee of our hospital and registered in Chinese Clinical Trial Registry (ChiCTR-OCH-11001332).
PiCCO monitoring
We used the PiCCO system (PULSION medical system, Germany) for the hemodynamic monitoring. This system is a transpulmonary thermodilution method that can provide information on volume status, including global end diastolic volume index (GEDVI), inrathoracic blood volume index (ITBVI) and central venous pressure (CVP). A central venous catheter was inserted into the internal jugular vein or subclavian vein, and PiCCO arterial catheter was inserted into the femoral artery. Then 10-15 mL normal saline at the temperature of <8 °C was injected into the central vein, and various hemodynamic parameters can be obtained through analysis of variations in blood temperature taken by the temperature sensor of the arterial catheter. The recordings of hemodynamic parameters were carried out at least every 8 hours. After the first measurement, fluid management and the use of vasoactive agents were instituted according the protocol of our institution. The first 8-hour was used as the study period and blood sampling for BNP was taken simultaneously at the first two transpulmonary thermodilution measurements.
Statistical analysis
Continuous variables were expressed as mean±standard deviation or median and interquartile range as appropriate. Categorical data were expressed as proportion. Pairwise correlation was used to test the correlation between BNP and cardiac parameters (CI, CVP, GEDVI and ITBVI). Because BNP values were not normally distributed, Spearman correlation analysis was used to analyze its correlation with GEDVI. ΔGEDVI was the difference between two measurements of GEDVI, its correlation with the difference of corresponding BNP values (ΔBNP) was analyzed. Two tailed test was used and a P value of <0.05 was considered statistically significant. Software Stata 10.0 (College Station, TX 77845 USA) was used for the statistical analysis.
Results
Clinical and hemodynamic characteristics of the 46 patients are shown in Table 1. The median age was 71 years old. More male patients were included with a male to female ratio of 30/16. The median APACHE II score was 23. The most common reason for PiCCO monitoring was septic shock (37%), followed by heart failure (19.6%), ARDS (21.7%) and hypovolemic shock (21.7%). Twenty-nine (63%) patients required mechanical ventilation and 39 (84.8%) required vasopressor/inotropes support due to hemodynamic instability. The median baseline serum creatinine was 142.5 μmol/L. The hemodynamic parameters were as follows: the median heart rate was 105 beats/min; the invasive arterial blood pressure was 126/66 mmHg; the median central venous pressure (CVP) was 9 mmHg; the median cardiac index (CI) was 3.47 L/min/m2; the median stroke index was 31 mL/m2; the median systemic vascular resistance index (SVRI) was 1,873 dyne∙sec/cm5∙m2; the median EVLWI was 9 mL/kg.
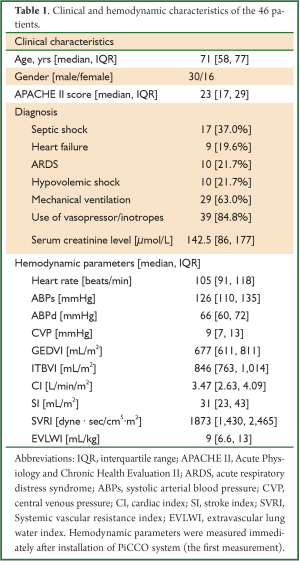
Full table
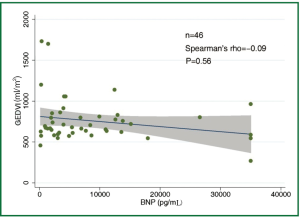
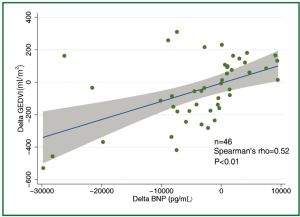
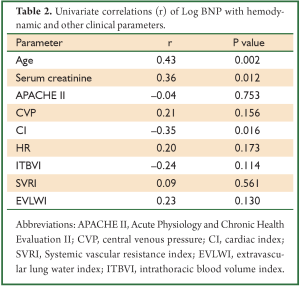
The concentration of BNP (median 4,602 pg/mL; IQR 1,988 to 12,439 pg/mL) was markedly elevated. However, the BNP concentration showed a weak and insignificant correlation with GEDVI (Figure 1; rho=-0.09, P=0.56). Each given GEDVI value was associated with wide ranges of BNP values. The differences of respective GEDVI (ΔGEDVI) and ΔBNP) between two measurements were obtained. ΔBNP showed significant correlation with ΔGEDVI (Figure 2; rho=0.52, P<0.01). Table 2 displays the correlation between logBNP with other variables. Age (r=0.43, P=0.002), serum creatinine (r=0.36, P=0.012) and CI (r=-0.35, P=0.016) were significantly correlated with logBNP. Other variables such as APACHE II score, CVP, HR, ITBVI, EVLWI and SVRI were not significantly correlated with logBNP. To exclude the influence of confounding factors on the relationship between BNP and GEDVI, multivatiate regression analysis was performed. The result showed that ΔBNP remained independently associated with ΔGEDVI (coefficient =0.012; 95% CI: 0.006-0.02).
Full table
Discussion
The study is among the few studies (11,18,19) that investigated the correlation between BNP concentration and parameters representative of cardiac preload. What is unique to our study is the use of transpulmonary thermodilution technique to evaluate cardiac preload (represented by GEDVI), which is thought to be more accurate than that estimated by filling pressures. However, our result showed that BNP concentration was not correlated with single measurement of GEDVI (rho=-0.09, P=0.56). When serial measurements of BNP concentrations and GEDVI were used for correlation analysis, ΔBNP showed significant correlation with ΔGEDVI (Figure 2; rho=0.52, P<0.01). The result was in line with other studies showing that BNP dropped in parallel with the reduction of cardiac preload (18,20). This is likely due to that many factors that (age, gender, renal function) cause wide variations in BNP in patients with similar volume status usually remain unaltered in a single patient, and serial measurement of BNP may be useful tool in monitoring hemodynamic changes. One clinical indication of our result is that, when a patient with initial high BNP concentration show reduced BNP after aggressive treatment, the volume status may be optimized. However, because there are wide variations in ΔGEDVI for any given value of ΔBNP, BNP concentration cannot replace invasive techniques (e.g., PiCCO, Swan-Ganz catheter) to quantitatively assess patient volume status.
The weak correlation between BNP and GEDVI is most probably due to the heterogeneity of included patients, which is an inherent limitation of studies conducted in mixed ICU. Previous studies identified strong correlation between BNP and volume status were those focused almost exclusively on patients with significant left ventricular dysfunction (21,22). However, a recent small study (17) focused on heterogeneous ICU patients also found significant correlation between NT-proBNP and GEDVI (r=0.61), and the author suggested NT-proBNP was a good indicator of cardiac preload. In contrast to our study, this study focused on patients receiving large volumes fluid replacement (>1,000 mL within 4 hours after hospitalization). This aggressive volume resuscitation may place cardiomyocytes at overly stretched status, in which BNP release correlates better with GEDVI. For patients with volume depletion, more fluid infusion (increased GEDVI) will simply put cardiomyocytes to their normal functional status but not overly stretch them, and BNP concentration will not increase proportionally to GEDVI.
Our secondary analysis showed that age, CI and serum creatinine were significantly correlated with BNP. Consistently with other studies (23-26), advanced age was found to be associated with increased BNP concentrations. This is thought to be due to age-related myocardial fibrosis and dysfunction. Even in normal subset of population (without cardiovascular, renal or pulmonary diseases), a correlation between age and BNP still exists. This has led to the call for age-specific reference range for BNP to classify heart failure (27). CI correlates significantly but weakly with BNP concentration (r=–0.35, P=0.016), which was consistent to the study by Forfia PR (11). CI is dependent on cardiac preload and contractility, and its value partly explains the fluctuation of BNP concentrations. Renal function has long been known as a determinant of circulating BNP level, because BNP is primarily excreted from kidney (28,29). Furthermore, renal dysfunction may coexist with higher arterial and systemic pressure, and greater ventricular mass, all factors contribute to BNP release. We have tried to reduce the impact of renal function on BNP levels by restricting study population to those with serum creatinine <200 μmol/L. However, the result indicates that even mild impairment of renal function contributes to the elevated BNP concentration.
In conclusion, the current study demonstrated that changes in BNP concentration was correlated with changes in GEDVI and serial measurement of BNP might be a useful tool for monitoring volume status. Other factors such as age, CI and renal function were also contributors of BNP concentration.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sudoh T, Kangawa K, Minamino N, et al. A new natriuretic peptide in porcine brain. Nature 1988;332:78-81.
- Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161-7.
- Karmpaliotis D, Kirtane AJ, Ruisi CP, et al. Diagnostic and prognostic utility of brain natriuretic Peptide in subjects admitted to the ICU with hypoxic respiratory failure due to noncardiogenic and cardiogenic pulmonary edema. Chest 2007;131:964-71.
- Richards AM, Crozier IG, Yandle TG, et al. Brain natriuretic factor: regional plasma concentrations and correlations with haemodynamic state in cardiac disease. Br Heart J 1993;69:414-7.
- Wei CM, Heublein DM, Perrella MA, et al. Natriuretic peptide system in human heart failure. Circulation 1993;88:1004-9.
- Mueller C, Scholer A, Laule-Kilian K, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med 2004;350:647-54.
- Tokola H, Hautala N, Marttila M, et al. Mechanical load-induced alterations in B-type natriuretic peptide gene expression. Can J Physiol Pharmacol 2001;79:646-53.
- Latour-Pérez J, Coves-Orts FJ, Abad-Terrado C, et al. Accuracy of B-type natriuretic peptide levels in the diagnosis of left ventricular dysfunction and heart failure: a systematic review. Eur J Heart Fail 2006;8:390-9.
- O’Neill JO, Bott-Silverman CE, McRae AT 3rd, et al. B-type natriuretic peptide levels are not a surrogate marker for invasive hemodynamics during management of patients with severe heart failure. Am Heart J 2005;149:363-9.
- Meaudre E, Jego C, Kenane N, et al. B-type natriuretic peptide release and left ventricular filling pressure assessed by echocardiographic study after subarachnoid hemorrhage: a prospective study in non-cardiac patients. Crit Care 2009;13:R76.
- Forfia PR, Watkins SP, Rame JE, et al. Relationship between B-type natriuretic peptides and pulmonary capillary wedge pressure in the intensive care unit. J Am Coll Cardiol 2005;45:1667-71.
- Della Rocca G, Costa MG, Coccia C, et al. Continuous right ventricular end-diastolic volume in comparison with left ventricular end-diastolic area. Eur J Anaesthesiol 2009;26:272-8.
- Huber W, Umgelter A, Reindl W, et al. Volume assessment in patients with necrotizing pancreatitis: a comparison of intrathoracic blood volume index, central venous pressure, and hematocrit, and their correlation to cardiac index and extravascular lung water index. Crit Care Med 2008;36:2348-54.
- Morgan P, Al-Subaie N, Rhodes A. Minimally invasive cardiac output monitoring. Curr Opin Crit Care 2008;14:322-6.
- Michard F, Alaya S, Zarka V, et al. Global end-diastolic volume as an indicator of cardiac preload in patients with septic shock. Chest 2003;124:1900-8.
- Malbrain ML, De Potter TJ, Dits H, et al. Global and right ventricular end-diastolic volumes correlate better with preload after correction for ejection fraction. Acta Anaesthesiol Scand 2010;54:622-31.
- Yamanouchi S, Kudo D, Endo T, et al. Blood N-terminal proBNP as a potential indicator of cardiac preload in patients with high volume load. Tohoku J Exp Med 2010;221:175-80.
- Knebel F, Schimke I, Pliet K, et al. NT-ProBNP in acute heart failure: correlation with invasively measured hemodynamic parameters during recompensation. J Card Fail 2005;11:S38-41.
- Januzzi JL, Morss A, Tung R, et al. Natriuretic peptide testing for the evaluation of critically ill patients with shock in the intensive care unit: a prospective cohort study. Crit Care 2006;10:R37.
- Johnson W, Omland T, Hall C, et al. Neurohormonal activation rapidly decreases after intravenous therapy with diuretics and vasodilators for class IV heart failure. J Am Coll Cardiol 2002;39:1623-9.
- Yoshimura M, Yasue H, Okumura K, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation 1993;87:464-9.
- Kazanegra R, Cheng V, Garcia A, et al. A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: a pilot study. J Card Fail 2001;7:21-9.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976-82.
- Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002;90:254-8.
- Rutten FH, Hoes AW. B-type natriuretic peptide assays for detecting heart failure in the elderly: same value as those in the younger? Int J Cardiol 2008;125:161-5.
- Maisel AS, Clopton P, Krishnaswamy P, et al. Impact of age, race, and sex on the ability of B-type natriuretic peptide to aid in the emergency diagnosis of heart failure: results from the Breathing Not Properly (BNP) multinational study. Am Heart J 2004;147:1078-84.
- Newton PJ, Betihavas V, Macdonald P. The role of b-type natriuretic peptide in heart failure management. Aust Crit Care 2009;22:117-23.
- Schou M, Dalsgaard MK, Clemmesen O, et al. Kidneys extract BNP and NT-proBNP in healthy young men. J Appl Physiol 2005;99:1676-80.
- Goetze JP, Jensen G, Møller S, et al. BNP and N-terminal proBNP are both extracted in the normal kidney. Eur J Clin Invest 2006;36:8-15.


