Selection for adjuvant chemotherapy in completely resected stage I non-small cell lung cancer: external validation of a Chinese prognostic risk model
Introduction
Surgical resection is the most common treatment for patients with localized non-small cell lung cancer (NSCLC) (1). Currently guidelines recommend adjuvant chemotherapy for patients with completely resected pathologic stage II NSCLC (2). No guidelines currently recommend additional chemotherapy for patients with completely resected pathologic stage I disease. However, the cancer specific survival in this best prognostic group is 60% to 80% with an expected 5 years recurrence rate of 30% to 55% (3-5).
The ability to sub-stratify survival within stage I is therefore important consideration as survival can be heterogeneous within this sub-group, and the ability to accurately predict sub-sets with poor outcomes despite stage I disease could be used to help select appropriate patients for adjuvant chemotherapy.
Recently, Liang et al. published a Chinese multi-institutional logistic regression derived model to predict post-operative survival in over 5,000 patients undergoing lung cancer surgery for all stages (6). The aim of our study is external validation of their published nomogram in a British cohort focusing on stages IA and IB to determine applicability in selection of adjuvant chemotherapy within stage I.
Materials and methods
Between 30 April, 2007 and 11 February, 2015, 1,442 consecutive patients who underwent resection for primary NSCLC at our institutions (Departments of Thoracic Surgery, Royal Brompton and Harefield NHS Trust Hospitals) were retrospectively analyzed from a prospectively collected database.
Patients’ data were collected including the following variables: sex, age, histologic subtype, type of operation, type of resection, number of lymph node stations sampled and pathologic tumor stage. Pathologic staging was characterized according to the seventh edition of the American Joint Committee on Cancer TNM staging system. Only patients diagnosed with NSCLC who underwent radical resection and pathologically stage I to II were included in this study. Neither neoadjuvant and/or adjuvant chemotherapy nor radiation therapy was performed for the patients during that period of time. We excluded 118 patients with carcinoid tumors (not in the original Chinese development set) and 86 patients without complete data on lymph node assessment leaving 1,238 patients for validation (validation cohort). Patients were followed up using the NHS tracing service through to February 2015.
Statistical analysis
Receiver operative characteristics curve (ROC) was calculated and compared with the original derivation cohort and the discriminatory ability was further quantified using survival plots by splitting our (external) validation cohort into three tertiles and Kaplan Meier plots were constructed and individual curves tested using Cox regression analysis on Stata 13 and R 3.1.2, respectively. The model performance for predicting outcome was evaluated by calculating the concordance index (C-index).
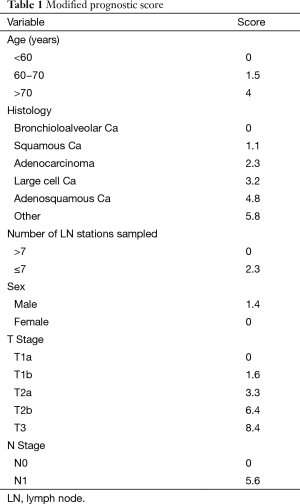
Prognostic scores were calculated using assigned variable values of the published Chinese coefficients (Table 1). The number of lymph node variable however had to be modified, as our center does not count individual nodes. Therefore, we substituted the number of lymph node stations rather than the number of actual nodes for the purposes of this work.
Full table
Results
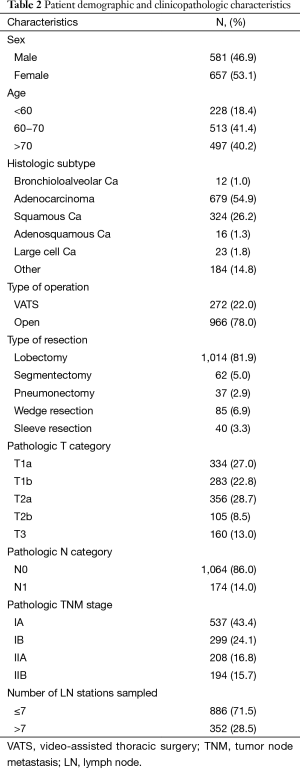
Of the 1,238 patients included, 657 (53.1%) were male and 581 (46.9%) were female. The median age at admission was 66 years (range, 13 to 89). The clinicopathologic characteristics of patients are summarized in Table 2.
Full table
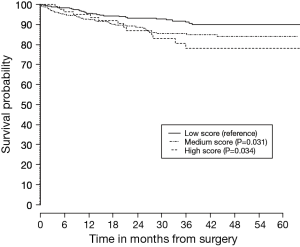
The mean prognostic score for all patients from stage IA to IIB was a mean (SD) of 9.95 (4.2). The ROC score comparing patients who died versus those that remained alive was 0.62 (95% CI: 0.58 to 0.67). When divided into prognostic score tertiles, survival discrimination remained evident for the entire validation cohort, as well as those for stage IA and IB alone. The P value comparing survival between the middle and highest score with baseline (low score) was P=0.031 and P=0.034 respectively (Figure 1). The C-index for overall survival was 0.61 [standard error (SE) of 0.02] and the C-index for stage I survival was 0.57 (SE of 0.03).
Discussion
After complete surgical resection, the prognoses expressed in terms of 5-year survival rates, are commonly accepted to be 60% to 80% for stage I and 30% to 50% for stage II, which are remarkably heterogeneous, leading to a number of investigators seeking to refine the prognostic ability with defined TNM categories (4). The most pertinent clinical application is the potential to sub select patients within stage I for consideration of adjuvant chemotherapy. We validated the risk model proposed by Liang et al. using a British cohort focusing on stages IA and IB and found reasonable discrimination within stage I (6).
We expected differences in clinicopathologic characteristics between the Chinese and British cohorts. The majority of patients in the Chinese cohort were male, compared with the almost balanced distribution in our cohort and the Chinese cohort (in general) was younger. We also noticed differences in the distribution of bronchioloalveolar and ‘other’ histologic subtypes (5.1% vs. 1% and 1.3% vs. 14.8%, respectively). Finally, the percentage of patients with stage I in the Chinese and validation cohorts were 47.0% and 67.5%, respectively. Despite these discrepancies between the two cohorts, the Chinese nomogram performed reasonably well.
On the application of the model the number of lymph nodes harvested was included as a variable in the risk model. This was a difficult measure, as the number of lymph nodes also varies greatly from one station to another and between laboratories and pathologists (7-11). Extraction modality of the nodal tissue sometimes leads to over or underestimation of accurate number of lymph nodes (12,13). We analyzed the number of sampled stations instead of the number of harvested lymph nodes. But more disconcerting is that the variable itself is a deterministic feature. What this means is that surgeons potentially have the “ability” to influence prognosis by the number of lymph nodes harvested. Most risk models are based on factors that cannot be “influenced”.
A significant number of patients with NSCLC undergoing curative resection ultimately die of systemic recurrence (14,15). The evidence supporting the use of adjuvant chemotherapy in stage II and III is broad and it has become the standard treatment for patients following complete resection within these stages (2,16,17). Despite conflicting researches, most studies demonstrate no benefits of adjuvant chemotherapy for stage IA NSCLC and currently it is not indicated for patients following complete resection (2,18-22). However, current trials that focus specifically on earlier stage indicate no clear consensus regarding the benefit of adjuvant chemotherapy for patients with stage IB. Both CALGB 9633 study and JABR 10 trial showed similar results and supported consideration of adjuvant chemotherapy only for large IB tumors. Nevertheless, this favor did not remain consistent in late follow-up reanalysis and the debate whether adjuvant chemotherapy is an effective application for stage IB NSCLC remains controversial (2,23-25). In scope of these debates, we aimed to validate the Chinese nomogram by focusing on stages IA and IB to determine applicability in selection of adjuvant chemotherapy within stage I.
In conclusion, our results of external validation suggested lower survival discrimination than reported by the original group; however discrimination between survival remained evident for stage I. The Chinese model could be useful for better estimation of survival of individual patients after surgery and for identifying subgroups of patients, especially in stage I, who may benefit from an adjuvant treatment strategy.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- al-Kattan K, Sepsas E, Fountain SW, et al. Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg 1997;12:380-4. [PubMed]
- Tanoue LT, Detterbeck FC. New TNM classification for non-small-cell lung cancer. Expert Rev Anticancer Ther 2009;9:413-23. [PubMed]
- Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res 2014;3:242-9. [PubMed]
- Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861-9. [PubMed]
- Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg 2014;97:385-93. [PubMed]
- Riquet M, Legras A, Mordant P, et al. Number of mediastinal lymph nodes in non-small cell lung cancer: a Gaussian curve, not a prognostic factor. Ann Thorac Surg 2014;98:224-31. [PubMed]
- Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol 2012;30:2823-8. [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [PubMed]
- Riquet M. Bronchial arteries and lymphatics of the lung. Thorac Surg Clin 2007;17:619-38. viii. [PubMed]
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50. [PubMed]
- Bria E, Milella M, Sperduti I, et al. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer 2009;66:365-71. [PubMed]
- Solomon B, Bunn PA Jr. Adjuvant chemotherapy for non-small cell lung cancer. Cancer Invest 2007;25:217-25. [PubMed]
- Johnson BE, Rabin MS. Patient subsets benefiting from adjuvant therapy following surgical resection of non-small cell lung cancer. Clin Cancer Res 2005;11:5022s-5026s. [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [PubMed]
- McElnay P, Lim E. Adjuvant or neoadjuvant chemotherapy for NSCLC. J Thorac Dis 2014;6 Suppl 2:S224-7. [PubMed]
- NSCLC Meta-analyses Collaborative Group, Arriagada R, Auperin A, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [PubMed]
- Imaizumi M; Study Group of Adjuvant Chemotherapy for Lung Cancer (Chubu, Japan). Postoperative adjuvant cisplatin, vindesine, plus uracil-tegafur chemotherapy increased survival of patients with completely resected p-stage I non-small cell lung cancer. Lung Cancer 2005;49:85-94. [PubMed]
- Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 2004;350:1713-21. [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [PubMed]
- Taguchi T. Clinical application of biochemical modulation in cancer chemotherapy: biochemical modulation for 5-FU. Oncology 1997;54 Suppl 1:12-8. [PubMed]
- Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010;28:29-34. [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [PubMed]