Adult patient with pulmonary agenesis: focusing on one-lung ventilation during general anesthesia
Introduction
Pulmonary agenesis is a rare congenital anomaly with a reported incidence of 1:15,000 autopsies. The pathogenesis of the disease is largely unknown and it can be classified into bilateral and unilateral forms. The bilateral form is incompatible with life. Herein, we reported a case of 61-year-old female presented to our hospital with pelvic mass and then underwent operation under general anesthesia. One-lung ventilation (OLV) and protective ventilation (PV) strategy was adopted during anesthesia. Unilateral pulmonary agenesis was diagnosed by chest computed tomography (CT) before operation.
Case presentation
A 61-year-old female presented to the hospital with cough and fever for one week. She also complained abdominal distension for 3 months. There was no chest pain, dyspnea or vomiting. Her medical history included repairing of atrial septal defect and mitral valve formation when she was 6-year-old. Past histories of her parents were unremarkable. She had been born at full term, and there had been no complications during pregnancy. On physical examination, she appeared alert, acyanotic, and breathed peacefully. However, the movement of her left chest decreased remarkably with breathing, and there was no breathing sound on this side. A grade II/VI systolic murmur was noted at the left sternal border. The second sound was higher than the first one on the pulmonary valve auscultation area. There was no tenderness or organomegaly on abdominal examination. The neurologic examination was unremarkable.
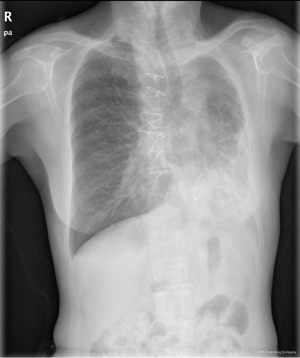
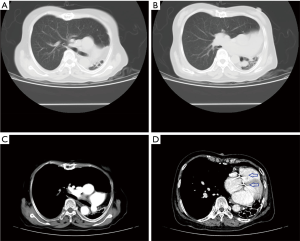
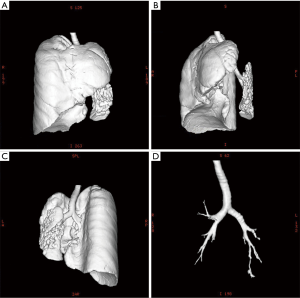
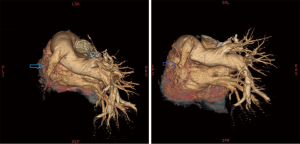
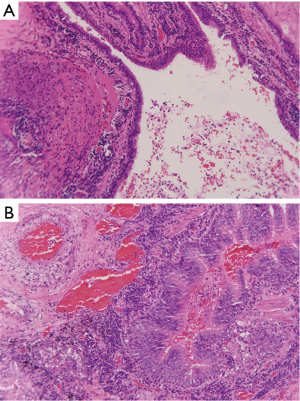
Chest X-ray performed at admission showed opacity on the bottom left thorax (Figure 1), together with deviation of the trachea and mediastinum to the left. Chest computed tomography (CT) showed collapse of the left lung with respiratory dead spaces and the right lung enlarged for compensation (Figure 2). A diagnosis of left lung agenesis was therefore established. CT 3D reconstruction showed dysplasia of the left lung with compensatory hypertrophy of the right lung, and the left pulmonary artery was thin with leftward deviation of the mediastinal structures (Figures 3,4). An electrocardiogram revealed sinus rhythm with a ventricular rate of 70 beats per minute, and there was a left-axis deviation. There were no changes in upper abdominal structures. Bronchoscopy revealed a blind-ending left grade three bronchus. Culture of protected specimen brush revealed pseudomonas aeruginosa which was susceptible to ciprofloxacin. Treatment with intravenous fluids and antibiotic IV ciprofloxacin (400 mg q12h) were initiated, and oxygen was given by nasal catheter. Histopathology showed that the right lung was hyperinflated with normal tissues. The left lung showed atrophy and some of the tissue presented bronchiectasis (Figure 5).
Laboratory investigations revealed mild elevation in arterial partial pressure of carbon dioxide (PaCO2 =53 mmHg). Doppler echocardiogram showed that the heart was positioned in right thorax. The atrioventricular level shunt and anomalous pulmonary venous return were not seen and pulmonary artery pressure was 58 mmHg. No genetic tests were performed yet.
Because of left lung collapse, the patient had to undergo OLV during the general anesthesia. A systematic review showed that PV could protect against the development of acute lung injury in patients who underwent major surgery. So prophylactic lung-protective mechanical ventilation strategy was adopted. To the best of our knowledge, there has been no case with lung agenesis undergoing OLV and PV being reported.
To prevent hypoxemia during OLV, we improved lung function before operation. The standard five pronged attack was used for pre-operative lung function improvement. It included reducing irritant exposure including smoking, bronchodilators for airway dilatation, mucolytic agent administration, chest physiotherapy to remove secretions and antibiotics to treat suspicious infection. Before operation the hemoglobin level was raised to 110 g/L by blood transfusion to improve oxygen supply. Double-lumen tube (DLT) was used for intubation, which was the standard method of lung isolation. Difficulty in selecting proper size is one of the disadvantages of DLT. With respect to the trachea 3D reconstruction, 35-Fr right-sided DLT was selected during anesthesia. Flexible fiber-optic bronchoscope was used to confirm the position of DLT. Once DLT was positioned at place, the adequacy of lung isolation was confirmed by ultrasonography. In the right lung, there was a to-and-fro movement at the pleural line that corresponds to tidal movement of the lung (lung sliding sign). The left lung showed the absence of lung sliding. Tidal volume was set to 6 mL/kg, corresponding to roughly 200 mL tidal volume for OLV. A positive end expiratory pressure (PEEP) of 5 cmH2O was given to the patient. The dynamic lung compliance was 120 mL/cmH2O and the airway resistance was 12 cmH2O. The pelvic lump was confirmed to be cervical carcinoma by histopathology. There were no postoperative pulmonary complications after surgery.
Discussion
Pulmonary agenesis, first reported by Brunner in 1963 is a rare congenital anomaly (1). Vitamin A deficiency, folic acid deficiency and salicylates were shown in experiments as the causes of pulmonary agenesis (2). The bilateral form is incompatible with life. In the absence of other malformations, unilateral pulmonary agenesis is compatible with normal life. The trachea continues directly into the main bronchus of the normally developed lung, and respiratory distress usually occurs due to retention of bronchial secretions and inflammations. Severe respiratory infection during infancy is common in these children and may lead to pneumonia and death before 5 years of age (3). Thus, patients usually present with cough, tachypnea, wheezing, respiratory distress, cyanosis or with chronic unproductive cough because of inflammations or bacterial colonization (4).
Its pathology has been categorized as per the classification by Schneider & Schwalbe: (I) agenesis-complete absence of a lung and bronchi, with no blood supply to the affected side; (II) aplasia-presence of a rudimentary bronchus with complete absence of parenchyma; and (III) hypoplasia-lobar agenesis and hypoplastic lung. Symptoms can manifest at birth as respiratory distress syndrome. Otherwise, patients may remain asymptomatic until adulthood when the defect is identified during routine examination for other purposes. Unilateral right lung agenesis is frequently associated with other congenital abnormalities like cardiovascular anomalies, and its prognosis is worse than the left side agenesis. This difference can be explained by a greater mediastinal shift for the right lung agenesis, leading to tracheal compression. Left lung agenesis is more common, and it typically results in compensatory growth of the right lung and ensuing herniation into the contralateral hemithorax (5).
OLV is frequently used in both adult and pediatric patients due to increasing use of thoracoscopy and video-assisted thoracoscopic surgery. However, the disadvantage of OLV is its association with more manipulation and damage of airway, leading to significant physiological derangements such as ventilation perfusion mismatching and early development of hypoxia (6,7). Development of hypoxaemia (arterial oxygen saturation <90%) caused by OLV can be explained by three interacting factors: (I) poor oxygenation and compromised ventilation, resulting in reduced oxygen stores; (II) dissociation of oxygen from haemoglobin; and (III) ventilation-perfusion mismatch (8). Although lung isolation has long been used in clinical practice, it remains controversial due to lack of specific and clear guidelines with respect to the choice of tube size, method of isolation and insertion, confirmation of correct placement, optimum FiO2 before and during OLV and the limits of acceptable degree of desaturation (9,10).
Mechanical ventilation is an essential supportive therapy to maintain gas exchange during general anesthesia, but inadequate ventilator settings can aggravate and even initiate lung damage in patients with healthy lungs at the onset of ventilation (11). Recent evidence showed that lung-protective mechanical ventilation using low tidal volume (6–8 mL/kg of predicted body weight), moderate PEEP (6–8 cmH2O), and recruitment maneuver is associated with improved postoperative outcome in patients undergoing major surgery (12). However, there are no recommendations on optimal ventilator settings in patients without lung injury during general anesthesia (13). Although lung-PV may be beneficial for the lung, it may impair the cardiovascular system, reduce venous return and cardiac output and require the use of fluids and vasopressors. Thus, the risks and benefits of lung-PV need to be balanced in each individual patient.
Conclusions
In aggregate, we reported the first case of OLV and PV strategy in patient with pulmonary agenesis during general anesthesia. The patient benefitted from PV without any postoperative pulmonary complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brunner S, Nissen E. Agenesis of the lung. Am Rev Respir Dis 1963;87:103-6. [PubMed]
- Meller CH, Morris RK, Desai T, et al. Prenatal diagnosis of isolated right pulmonary agenesis using sonography alone: case study and systematic literature review. J Ultrasound Med 2012;31:2017-23. [PubMed]
- Gabarre JA, Galindo Izquierdo A, Rasero Ponferrada M, et al. Isolated unilateral pulmonary agenesis: early prenatal diagnosis and long-term follow-up. J Ultrasound Med 2005;24:865-8. [PubMed]
- Jentzsch NS. Unilateral pulmonary agenesis. J Bras Pneumol 2014;40:322-4. [PubMed]
- Hasegawa T, Oshima Y, Maruo A, et al. Pediatric cardiothoracic surgery in patients with unilateral pulmonary agenesis or aplasia. Ann Thorac Surg 2014;97:1652-8. [PubMed]
- Mayhew PD, Pascoe PJ, Shilo-Benjamini Y, et al. Effect of One-Lung Ventilation With or Without Low-Pressure Carbon Dioxide Insufflation on Cardiorespiratory Variables in Cats Undergoing Thoracoscopy. Vet Surg 2015;44 Suppl 1:15-22. [PubMed]
- Purohit A, Bhargava S, Mangal V, et al. Lung isolation, one-lung ventilation and hypoxaemia during lung isolation. Indian J Anaesth 2015;59:606-17. [PubMed]
- Reinius H, Borges JB, Fredén F, et al. Real-time ventilation and perfusion distributions by electrical impedance tomography during one-lung ventilation with capnothorax. Acta Anaesthesiol Scand 2015;59:354-68. [PubMed]
- Şentürk M, Slinger P, Cohen E. Intraoperative mechanical ventilation strategies for one-lung ventilation. Best Pract Res Clin Anaesthesiol 2015;29:357-69. [PubMed]
- Tusman G, Böhm SH, Suarez-Sipmann F. Dead space during one-lung ventilation. Curr Opin Anaesthesiol 2015;28:10-7. [PubMed]
- Bender SP, Paganelli WC, Gerety LP, et al. Intraoperative Lung-Protective Ventilation Trends and Practice Patterns: A Report from the Multicenter Perioperative Outcomes Group. Anesth Analg 2015;121:1231-9. [PubMed]
- Zhang Z, Hu X, Zhang X, et al. Lung protective ventilation in patients undergoing major surgery: a systematic review incorporating a Bayesian approach. BMJ Open 2015;5:e007473. [PubMed]
- Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015;351:h3646. [PubMed]


