Clinicopathologic correlates of postoperative N1 or N2 nodal upstaging in non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death globally (1). Non-small cell lung cancer (NSCLC), which in earlier stages is treatable by surgical resection. To date, anatomic TNM stage is considered pivotal in the prognosis of NSCLC (2) and is the most important therapeutic determinant, according to National Comprehensive Cancer Network (NCCN) guidelines (v6.2015). The presence or absence of nodal metastases is critical in deciding whether chemotherapy and radiation should be administered in conjunction with surgical resection.
Chest computed tomography (CT) and positron emission tomography (PET)/CT scans are useful for preoperative TNM staging (3,4). Invasive methods, such as fine-needle aspiration (FNA) with ultrasound (US) guidance (endobronchial or esophageal endoscopic routes) or mediastinoscopy, may also be utilized to improve the accuracy of preoperative nodal staging (5). However, the added cost of invasive procedures is not warranted if surgery is supported by chest CT or PET/CT findings alone (6). At our institution, complete resection of tumors and enlarged lymph nodes relies entirely on noninvasive clinical staging (as above) if feasible.
Given this approach, there is the distinct possibility that preoperative clinical staging and postoperative pathologic staging may conflict, once surgical specimens are thoroughly examined. Consequently, our aim was to identify clinical and pathologic characteristics that may influence nodal upstaging of this sort, comparing circumstances in which tumors were eventually upstaged to pathologic N1 (pN1) or N2 (pN2) with unchanged same-stage counterparts scenarios.
Patients and methods
Patients
Between January, 2011 and December, 2014, 634 patients at Seoul St. Mary’s Hospital in Korea were diagnosed with NSCLC and underwent surgical resection. Of this population, 140 patients were staged as pN1 or pN2. After excluding 37 patients who received neoadjuvant chemotherapy prior to surgery, 103 patient charts were reviewed retrospectively. In instances of postoperative nodal upstaging patients were assigned to group A, whereas patients whose pre- and postoperative nodal staging remained unchanged were assigned to group B. Clinical characteristics of the two groups and respective pathologic features were compared. The TNM classification system of the American Joint Committee on Cancer was applied for staging purposes. All clinical specimens were examined by certified pathologists, and their observations were duly recorded. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital at the Catholic University of Korea.
Preoperative and intraoperative lymph node staging
Preoperative clinical staging was achieved by contrast-enhanced chest CT scan and F-18-fluorodeoxyglucose (FDG)-PET/CT scan. Malignancy was signaled by a short-axis nodal diameter >10 mm on CT scan and by nodal uptake of FDG beyond surrounding mediastinal structures, unless benign nodal calcifications were noted or nodes on unenhanced CT images were highly attenuated, with distinct margins (4). Surgery was elected if complete resection was feasible, despite questionable nodal status on chest CT and PET/CT scans; and systemic lymph node dissection was routinely performed to accurately stage patients postoperatively. More than three stations of mediastinal nodes were dissected, including paratracheal and subcarinal nodes on the right and para-aortic, subaortic, and subcarinal nodes on the left.
Statistical analysis
Clinicopathological characteristics of group A (upstaged) and group B (unchanged) were compared. Student’s t-test or Wilcoxon rank-sum test was used to assess continuous variables, and χ2 or Fisher’s exact test was applied for categorical variables. Multivariate logistic regression was performed to analyze factors that impacted postoperative nodal upstaging. Statistical significance was set at P<0.05.
Results
Of 103 patients staged as pN1 or pN2, upstaging occurred in 59 patients (group A); whereas 44 patients (group B) retained initial nodal staging. In group A, 35 of the 59 patients were upstaged to pN1 from clinical N0 (cN0), 15 to pN2 from cN0, and 9 to pN2 from cN1.
Clinical characteristics of the two groups were similar (Table 1). However, mean age in group A was comparatively lower (61.6 vs. 68.4 years; P<0.001); there were more women in group A by comparison (47.5% vs. 15.9%; P=0.001); and average patient smoking history in group A (vs. group B) was shorter (12.2 vs. 28.8 pack years; P<0.001). Tumor distribution (lobe involved) did not differ by group, and peripherally located tumors predominated in both groups. Extent of resection (generally standard) was similar for both groups (P=0.0180). In two instances, patients of group B underwent limited resections (one wedge resection and one segmentectomy) due to poor pulmonary function, but full systemic lymph node dissection was done in each case. Average counts of lymph nodes dissected in groups A and B were comparable (16.6±7.8 vs. 19.4±10.5; P=0.115). Video-assisted thoracoscopic surgery (VATS) was often performed, although significantly more so in group A (P=0.006) given the high number of patients with cN0 or cN1 staging. Mean maximum standardized uptake value (SUVmax) of PET/CT scans was higher in group B subjects (7.3±3.6 vs. 10.4±4.3; P=0.001).
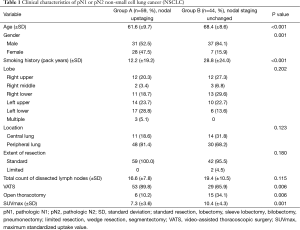
Full table
Pathologic characteristics of groups A and B are summarized (Table 2), showing no difference in average tumor size (3.4±1.8 vs. 3.8±1.9 cm; P=0.298). A majority of tumors in group A (vs. group B) were adenocarcinomas (84.7% vs. 38.6%; P<0.001). Squamous cell carcinoma predominated in group B (45.5%). Similar group staging distributions (P=0.812) were noted, and both groups were comparable in terms of pleural (P=0.133), lymphatic (P=0.461), or vascular invasion (P=0.163). In group A, the epidermal growth factor receptor (EGFR) mutation rate (36/59, 61.0%) was significantly higher than that of group B (11/44, 25.0%; P=0.001). Micropapillary (MPC) and lepidic (LPC) histologic components, widely acknowledged as prognostic biomarkers in adenocarcinoma, were compared as well. Both MPC and LPC positivity (i.e., ≥5% of tumor composition) were significantly more frequent in group A (61% vs. 13.6% and 59.3% vs. 22.7%, respectively; both, P<0.001).

Full table
In addition to the clear predilection for adenocarcinoma in group A (vs. group B), some factors were distinctly associated with adenocarcinoma, as opposed to other types of NSCLC, particularly younger age, female gender, shorter smoking history, lower SUVmax, and positivity of biomarkers (EGFR, MPC, and LPC) (1). The 67 patients diagnosed with adenocarcinoma were then compared by group (Table 3), showing that age, SUVmax, EGFR mutation, and LPC positivity did not differ between groups. However, patients in group A (vs. group B) with adenocarcinoma were significantly more often female (56.0% vs. 23.5%; P=0.026), with shorter smoking history (8.3±14.3 vs. 21.7±21.7 pack years; P=0.027) and greater likelihood of MPC positivity (72% vs. 35.3%; P=0.008).

Full table
Multivariate analysis (via logistic regression model) was performed to gauge potential influences on nodal upstaging (Table 4). Factors linked with adenocarcinoma (female gender, smoking history, and MPC positivity), as well as tumor histotype (whether adenocarcinoma or not), served as covariates. Ultimately, MPC positivity [odds ratio (OR) =4.735; P=0.013] was identified as the sole parameter significantly impacting nodal upstaging.
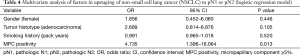
Full table
Discussion
NSCLC accounts for most lung cancers and is treatable surgically in early stages. As the most important determinant of treatment and prognosis, accurate TNM staging is essential before and after surgery. However, preoperative clinical staging of nodes by imaging and postoperative pathologic staging of nodal dissections are bound to differ in some instances. One particular source citing carcinoembryonic antigen (CEA), SUVmax, and tumor size as factors predictive of pathologic upstaging (7) is supported by other studies that confirm the predictive values of CEA and SUVmax in nodal upstaging (3,8). Herein, we have addressed the nature and character of tumors that typically produce occult lymph node metastases, comparing tumors that were upstaged with same-stage tumors, unchanged in status postoperatively.
At many institutions, surgeries are performed without benefit of invasive lymph node evaluations if chest CT and PET/CT scans offer satisfactory assessments (3,6,8). This is done because systemic lymph node dissection is often appropriate for either N1 or N2 nodal staging (as stipulated by NCCN guidelines) but is not indicated for N3 disease. Especially at N1 stage or in single-station N2 staging, surgical resection may improve patient prognosis (6,9). Our institution favors this strategy, encouraging surgery as primary treatment in patients with N1 or resectable single-station N2 staging by chest CT and PET/CT scans. Although patients of group A were upstaged postoperatively from cN0 or cN1 to pN1 or pN2, others clinically staged as N1 or N2 in group B who remained unchanged conceivably may have had more advanced or aggressive disease, presenting as single station or non-bulky lymph node metastasis on imaging studies. Still, the two patient groups did not differ significantly in terms of final pathologic staging, so between-group comparisons seem reasonable.
Invasive adenocarcinomas are inherently heterogeneous, displaying a mixture of histologic elements. The primary growth patterns (acinar, papillary, micropapillary, solid, and lepidic) may serve as subtypes through semi-quantitation (5% thresholds) (10). Various studies have indicated that subtyping generally reflects the prognosis of adenocarcinoma (11-15). However there are cases in which characteristics of tumor change depending on whether a small amount of histologic pattern is included or not in the tumor. MPC and LPC are commonly identified in many studies delineating the relationship between tumor histology and patient prognosis (16-22). Because our analysis was confined to pN1 or pN2 staging after radical surgery and the number of patients was limited, the value of classifying adenocarcinomas by subtype was questionable. Instead, the impact of MPC and LPC positivity was assessed.
In patients of group A, adenocarcinoma clearly predominated. As a result, the clinicopathologic factors that differed by group were largely those associated with adenocarcinoma (1). In between-group comparisons of the 67 patients with adenocarcinoma, gender, smoking history, and MPC positivity emerged as significant parameters. Using the latter as covariates, in conjunction with tumor histotype (whether adenocarcinoma or not) the entire cohort was included in multivariate analysis. MPC positivity was ultimately confirmed as the sole significant variable in nodal upstaging (albeit restricted to adenocarcinoma), with a substantial OR of 4.735.
A number of studies have linked a poorer prognosis with MPC positivity (16,20,21). In one investigation, small amounts of MPC in tumors were associated with tumor recurrence, and the recurrence rate was comparatively higher after limited resection of MPC-positive tumors (23). This implies that MPC may have a singularly profound tumor effect, regardless of other components. In addition, there are studies attributing augmented tumor invasiveness and lymph node metastasis to MPC elevations (16,22,24). Accordingly, the thrust of this study (i.e., MPC positivity promotes occult lymph node metastasis) is similar. Given that MPC-dependent nodal metastasis escapes ready detection by chest CT and PET/CT scans, confirming or excluding MPC positivity at the time of intraoperative frozen section seems imperative. Unfortunately, this objective is limited by current methods and applicable requirements (25). If MPC positivity must be await postoperative confirmation, further surgery may be needed to ensure proper treatment, namely completion lobectomy and systemic lymph node dissection.
This study has several acknowledged limitations, the most obvious being its retrospective design, the single-institution accrual of data, and the relatively small number of patients recruited over a period of just 4 years. However, the efforts made were consistent, involving similar preoperative evaluation and treatment plans and reproducible surgical techniques. Still, our preoperative nodal staging was subject to inaccuracy due to a lack of invasive procedures, which were prohibitive in this context. Clinical staging of nodes was thus limited to available imaging methods. Further verification of outcomes are warranted here, using combined invasive and imaging assessments.
In conclusion, a significant relationship between MPC positivity and occult lymph node metastasis was demonstrated in patients with NSCLC at pN1 or pN2 stage. If MPC positivity is confirmed postoperatively in such patients, completion lobectomy and systemic lymph node dissection are appropriate and may necessitate further surgery.
Acknowledgements
The manuscript has been edited by native English-speaking experts at BioMed Proofreading, LLC.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Edge SB, Byrd DR, Compton CC, et al, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010.
- Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014;98:217-23. [PubMed]
- Shin S, Kim HK, Choi YS, et al. Prognosis of unexpected and expected pathologic N1 non-small cell lung cancer. Ann Thorac Surg 2013;96:969-75; discussion 975-6. [PubMed]
- Kirmani BH, Rintoul RC, Win T, et al. Stage migration: results of lymph node dissection in the era of modern imaging and invasive staging for lung cancer. Eur J Cardiothorac Surg 2013;43:104-9; discussion 109-10. [PubMed]
- Mehran R. The role of surgery in patients with clinical n2 disease. Thorac Surg Clin 2013;23:327-35. [PubMed]
- Yamazaki K, Yoshino I, Yohena T, et al. Clinically predictive factors of pathologic upstaging in patients with peripherally located clinical stage IA non-small cell lung cancer. Lung Cancer 2007;55:365-9. [PubMed]
- Haruki T, Aokage K, Miyoshi T, et al. Mediastinal nodal involvement in patients with clinical stage I non-small-cell lung cancer: possibility of rational lymph node dissection. J Thorac Oncol 2015;10:930-6. [PubMed]
- Moreno AC, Morgensztern D, Boffa DJ, et al. Treating locally advanced disease: an analysis of very large, hilar lymph node positive non-small cell lung cancer using the National Cancer Data Base. Ann Thorac Surg 2014;97:1149-55. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol 2013;8:612-8. [PubMed]
- Zhang J, Wu J, Tan Q, et al. Why do pathological stage IA lung adenocarcinomas vary from prognosis?: a clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J Thorac Oncol 2013;8:1196-202. [PubMed]
- Hung JJ, Yeh YC, Jeng WJ, et al. Predictive value of the international association for the study of lung cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J Clin Oncol 2014;32:2357-64. [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg 2014;98:453-8. [PubMed]
- Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. [PubMed]
- Sasada S, Nakayama H, Miyata Y, et al. Comparison of malignant grade between pure and partially invasive types of early lung adenocarcinoma. Ann Thorac Surg 2015;99:956-60. [PubMed]
- Zhang J, Liang Z, Gao J, et al. Pulmonary adenocarcinoma with a micropapillary pattern: a clinicopathological, immunophenotypic and molecular analysis. Histopathology 2011;59:1204-14. [PubMed]
- Yeh YC, Wu YC, Chen CY, et al. Stromal invasion and micropapillary pattern in 212 consecutive surgically resected stage I lung adenocarcinomas: histopathological categories for prognosis prediction. J Clin Pathol 2012;65:910-8. [PubMed]
- Sumiyoshi S, Yoshizawa A, Sonobe M, et al. Pulmonary adenocarcinomas with micropapillary component significantly correlate with recurrence, but can be well controlled with EGFR tyrosine kinase inhibitors in the early stages. Lung Cancer 2013;81:53-9. [PubMed]
- Chao L, Yi-Sheng H, Yu C, et al. Relevance of EGFR mutation with micropapillary pattern according to the novel IASLC/ATS/ERS lung adenocarcinoma classification and correlation with prognosis in Chinese patients. Lung Cancer 2014;86:164-9. [PubMed]
- Moon Y, Kim KS, Sung SW, et al. Correlation of histological components with tumor invasion in pulmonary adenocarcinoma. World J Surg Oncol 2014;12:388. [PubMed]
- Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. [PubMed]
- Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 2010;34:1155-62. [PubMed]
- Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ≤ 3 cm: accuracy and interobserver agreement. Histopathology 2015;66:922-38. [PubMed]


