Otorhinolaryngological aspects of sleep-related breathing disorders
Introduction
Sleep-related breathing disorders (SRBD) encompass a wide range of conditions in which there is recurrent partial or complete cessation of breathing, including simple snoring, upper airway resistance syndrome, central sleep apnoea-hypopnoea syndrome, obesity-related sleep hypoventilation syndrome and obstructive sleep apnoea-hypopnoea syndrome (OSAHS) (1). This spectrum of disorders is common and not only negatively impacts on patient health but also induces a significant social and economic burden (2-4). Epidemiological studies indicate that the prevalence of snoring is 25-50% for middle-aged men whilst OSAHS affects 2-4% of males and 1-2% of females (5-7). SRBD, particularly OSAHS, can increase the risk of cardiac arrhythmias, pulmonary and systemic hypertension, myocardial infarction, type 2 diabetes mellitus, cerebrovascular accidents, impaired cognition and road traffic accidents (8-12). Furthermore, there is evidence that moderate-to-severe OSAHS independently correlates with a large increased risk of all-cause mortality (13). The pathophysiology of SRBD is complex and multifactorial, with a single cause rarely identified. Associations include obesity, increased neck circumference, craniofacial abnormalities and anatomical variations (e.g., retrognathia, macroglossia, nasal polyposis), hypothyroidism, acromegaly, family history, alcohol or sedative intake and body position (14).
This review will focus on the management of the two most common forms of SRBD, simple snoring and OSAHS.
Clinical evaluation
The clinical history is attained from both the patient and the partner, if present. In many cases, patients attend with a recording to outpatient clinic which can be of value. The principle symptom is often of socially embarrassing snoring; severe snoring can be as loud as 90 dB. The partner may also elucidate concerns of apnoeic episodes. Patients may also complain of daytime somnolence. Further direct questioning is required to ascertain how refreshed the patient feels in the morning, the presence of morning headaches, night sweats, palpitations during the night, choking sensation, restless sleep, acid reflux, decreased libido alongside impaired concentration and memory. A useful adjunct in this regard is the ubiquitous use of the Epworth Sleepiness Scale questionnaire, whereby higher scores, particularly above 10, are correlated with OSAHS (Table 1) (15). It is also important to ascertain any associated rhinological symptoms, mouth breathing and medical history to include alcohol and sedative intake, as these act as muscle relaxants and worsen symptoms. Nasal problems such as polyposis, alar collapse and a deviated septum can contribute significantly to SRBDs and form an important part of treatment non-compliance with nasal continuous positive airway pressure (nCPAP). It is thus important to fully delineate and examine for this subset of conditions (16). A full assessment of patient health status and past medical history is also warranted to assess co-morbidities and rule out acromegaly or hypothyroidism, for example.

Full table
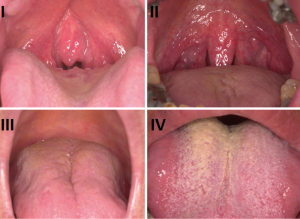
Clinical examination serves to assess the upper airway including the nasal and oral cavities alongside the anatomical segments of the pharynx and larynx. A general inspection is initially invaluable however to assess for features such as dental malocclusion, retrognathia, craniofacial abnormalities and body habitus. Body mass index (BMI) and neck collar size should be measured. Nasal examination with anterior rhinoscopy, misting testing and rigid endoscopes will enable evaluation for rhinological factors contributing to SRBDs. Visualisation of the oral cavity and oropharynx provides information regarding the grade of the palatine tonsils, the dimensions of the soft palate and uvula, and evidence of redundant pharyngeal tissue. In addition, clinicians can evaluate tongue position using Friedman or Mallampati gradings, which have been shown to correlate strongly with predicting OSAHS (Figure 1) (18-20). Furthermore, there is evidence that patients with Friedman tongue position 1 are more likely to benefit from palatal surgery whilst those with tongue positions 3 or 4 are unlikely to benefit (21). The optimal examination technique is flexible nasopharyngolaryngoscopy and allows visualisation and assessment of the pharynx and larynx including the tongue base. Furthermore, this procedure, although somewhat dependent on clinician experience, allows a dynamic evaluation of the upper airway which can be invaluable, particularly with additional steps such as simulated snoring or Muller’s manoeuvre, to assess the level of collapse. Despite evidence that these manoeuvres correlate with Epworth scores and sleep study findings, there are difficulties in standardising this effectively subjective assessment (22-24).
Investigations
The gold standard investigation to differentiate between simple snoring and OSAHS is hospital-based polysomnography. Due to financial constraints, patient preference and availability, ambulatory sleep studies are often performed. Recorded parameters include oxygen saturation, nasal and oral airflow, respiratory effort (via chest and abdominal movements) and sleep architecture, the latter of which is not possible with home or ambulatory sleep studies (25). Objective values often analysed from these studies include apnoea/hypopnoea index (AHI), mean oxygenation and oxygen desaturation index. An apnoea has been defined as a cessation of airflow for at least 10 seconds whilst a reduction in tidal volume or vital capacity by at least 30% is a hypopnoeic episode (1). Moreover, AHI serves as a marker of severity with mild [5-15], moderate [15-30] and severe OSAHS [more than 30] delineated by the index score (1). These markers should of course be interpreted in light of the patient’s age, symptoms and co-morbidities (14).
Further investigations have been proposed ranging from imaging, acoustic analysis, pressure transducers and sleep nasendoscopy but all have limitations and thus have not been universally accepted (26).
Drug-induced sedation endoscopy (DISE) or sleep nasendoscopy has been tested most thoroughly since its introduction by Croft and Pringle in 1991 (27). The main criticism remains that drug-induced sleep differs from natural physiological sleep alongside the inherent subjectivity in assessment and lack of standardised grading systems. This is countered by the suggestion that these drugs would affect different segments equally and thus still allow evaluation of obstruction at each anatomical level. Alongside this, recent studies have confirmed superiority to awake assessment by flexible nasopharyngolaryngoscopy in outpatients and correlation with AHI, mean oxygen desaturation alongside surgical outcomes with good inter-rater reliability (26,28-33). Moreover the recent European congress meeting has met in an attempt to standardise nomenclature and data capture from these procedures (34). As a corollary to this standardisation, the advent of a neurophysiological (bispectral index) monitoring device may indicate at which juncture DISE should be performed, potentially allowing for the development of clearer protocols (28).
Sleep physiology and polysomnography
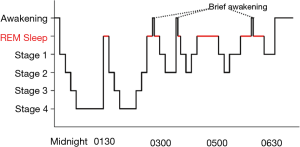
Sleep is a natural periodic state of rest characterised by reduced or absent consciousness, reduced sensory activity and voluntary muscle inactivity. Although humans spend about one third of their lives asleep and deprivation can lead to serious physiological consequences, the function of sleep remains to be fully elucidated. Typical sleep architecture is comprised of two types: non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. A normal sleep episode consists of a cycle between NREM and REM sleep, with the majority in NREM stage and the cycle varying in length from 70 to 100 minutes earlier and 90 to 120 minutes later in the night (Table 2, Figure 2).

Full table
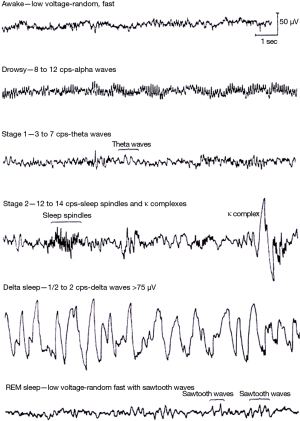
Slow-wave or NREM sleep is characterised by four stages with distinct neurophysiological features. Its duration and frequency decreases with age. Stage 1 sleep is the transition between sleep and wakefulness and lasts 1-15 minutes with a reduction of alpha waves, indicative of wakefulness, transitioning to low voltage, mixed frequency waves. Stage 2 sleep can last 10-25 minutes and increases in length throughout the sleep cycle constituting 45-55% of total sleep time. Electroencephalograms (EEG) demonstrate sleep spindles, K complexes and relatively low-voltage, mixed frequency activity (Figure 3). Stages 3 and 4 sleep show low frequency delta waves with reducing sleep spindles. Snoring will tend to occur in the latter stages. The American Academy of Sleep Medicine have recently reclassified NREM into 3 stages (N1, N2, N3) which correlate to stage 1, stage 2 and stages 3/4 respectively.
REM sleep is defined by the presence of desynchronised brain wave activity (similar to wakefulness), muscle atonia and bursts of REM. The length of REM sleep increases throughout the sleep cycle whilst NREM stages 3 and 4 decrease. The numerous physiological changes and comparisons between NREM and REM sleep are outlined in Table 2.
Ventilation and respiratory flow change during sleep and become increasingly faster and more erratic during REM sleep. In addition, the efficacy of adaptive responses is reduced during sleep. The cough reflex, hypoxic ventilatory drive and arousal response to respiratory resistance are all suppressed in various stages of the sleep cycle, in part due to reduced muscle tone.
Polysomnography is a comprehensive recording of biophysiological changes during sleep with recorded parameters including oxygen saturation, nasal and oral airflow, respiratory effort (via chest and abdominal movements) and sleep architecture, the latter of which is not possible with home or ambulatory sleep studies (25). Numerous attachments are therefore required for electroencephalography, electro-oculography, electromyography, electrocardiography, pulse oximetry, recording of snoring, body position, leg muscle activity, pressure transducers for nasal and oral airflow alongside belts to assess chest and abdominal movements. Polysomnography allows the diagnosis of both sleep and movement disorders (35).
Objective values often analysed from these studies include AHI, mean oxygenation and oxygen desaturation index. An apnoea has been defined as a cessation of airflow for at least ten seconds whilst a reduction in tidal volume or vital capacity by at least 30% is a hypopnoeic episode (1). Moreover, AHI serves as a marker of severity with mild [5-15], moderate [15-30] and severe OSAHS [more than 30] delineated by the index score (1). These markers should of course be interpreted in light of the patient’s age, symptoms and co-morbidities (14).
Management
Limitations
The lack of a high level of evidence has been highlighted by numerous authors for this topic but also surgery in general. Many therefore recommend larger scale clinical studies (14,36). However, an oft-neglected caveat is the lack of standardisation of nomenclature not only in DISE but also in the definition of success and outcomes (14,36-38). For example, many continue to use AHI as a single ‘end-point’ demarcating success but a combination of patient-centred and objective outcomes would be preferable. As a corollary to this, the technique modifications amongst similar surgical procedures is myriad and makes comparison difficult and of little clinical value. A recent Cochrane review underlined this issue and further work needs to be done to ensure improved data collation and analysis along with formulation of tangible and answerable trial hypotheses (39,40).
Management strategies are generally primary and adjunctive in that they may be used alone or in combination with other treatment modalities.
Non-surgical
Lifestyle modifications can be sufficient to reduce snoring significantly. Reducing body weight, alcohol intake and positional therapy (by avoiding sleep positions precipitating symptoms such as supine) are of value. Recent studies have indicated that sleep position therapy can be highly efficacious (41-44). Medications such as those to aid in weight loss or treat contributing conditions such as allergic rhinitis or hypothyroidism may also be of use. However, there is no convincing evidence that these alone will treat OSAHS effectively (45,46).
Appliances used in treating SRBD include nasal dilators, mandibular advancement splints (MAS) and nCPAP. Nasal dilators are reserved for those patients suffering from simple snoring and nasal obstruction; these patients may benefit from reconstructive nasal surgery if there is a significant improvement. MAS efficacy can be predicted by DISE and function by protruding the hyoid bone anteriorly along with the mandible, contracting genioglossus and thus increasing the retroglossal distance (47). The main drawback is patient compliance due to discomfort and, as with all appliances, the requirement for use every night, resulting in compliance in the range of 60-70%. MAS is contraindicated in those with uncontrolled epilepsy, poor dentition and edentulous patients (47-51). In keeping with UK national guidelines, the current treatment of choice for moderate to severe OSAHS is CPAP, most frequently nCPAP although autotitrating CPAP (which responds to the individual’s airflow patterns) and bilevel positive airway pressure (BiPAP) have also been used. Studies demonstrate that patients overestimate their compliance, with reported rates as low as 40-60% (52,53). These patients should be diligently assessed for any pathology contributing to non-compliance and/or higher CPAP pressure such as CPAP rhinitis, deviated septum, nasal polyposis or tonsillar hypertrophy. Further complications include dermatitis, rhinitis, epistaxis, aerophagia and barotrauma. Addressing these factors either medically or surgically may facilitate the use of CPAP with lower pressure requirements.
Surgical
The primary aim of surgery is either to bypass upper airway obstruction or to increase the upper airway anatomical dimensions. Despite the limitations outlined above and criticisms regarding possible overenthusiastic use of surgery, there is evidence that surgical outcomes are good if patients are selected diligently (38,54-61). Patient selection is therefore integral in ensuring successful outcomes and surgical options should be offered judiciously, with an underlying principle of site-specific surgery. Surgery is generally reserved for patients with simple snoring or mild OSAHS. For moderate to severe OSAHS, surgery is only considered in patients who have failed CPAP. In these cases, surgery may serve to reduce CPAP pressures and hence patient compliance. Patients with a high BMI tend to do less well. To allow effective patient selection and site-specific surgery, DISE is invaluable (27,29,33,62). It is worth highlighting that although in some cases a single procedure can resolve symptoms, in the main, patients display multilevel obstruction requiring careful assessment and treatments.
Nasal surgery, including septoplasty, turbinate reduction, endoscopic sinus surgery and septorhinoplasty or nasal valve surgery, can be efficacious for simple snorers and in facilitation of CPAP usage by reducing pressure requirements along with patient discomfort (16,63-65).
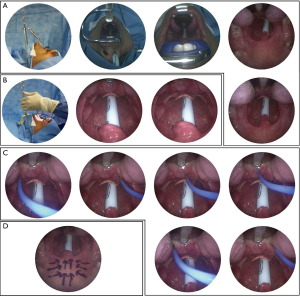
Palatal surgery incorporates a wide range of procedures and is the most common surgery performed for these patients. Conservative procedures are usually initially favoured over more aggressive surgery. Minimally invasive surgeries maintain the anatomy of the soft palate but aim to scar or stiffen the soft palate by, for example, chemical injections (e.g., sodium tetradecyl sulphate ‘injection snoreplasty’), palatal implants or radiofrequency treatments. The evidence base and long term efficacy of these procedures is questionable, particularly in the OSAHS group (66-68). The most promising recent research, including a meta-analysis, has however highlighted the efficacy of radiofrequency applications to the soft palate for both simple snorers and OSAHS patients (38,55,69). Radiofrequency thermoablation has few complications although ulceration and fistula formation has been reported, with NICE guidance highlighting this safety profile (68). In addition, it can be performed under local or general anaesthetic (Figure 4) (38,55,69-72). Radical palatal surgery involves altering the anatomy of the soft palate by removing excess tissue (e.g., uvula, soft palate, redundant pharyngeal mucosa, tonsils) alongside inducing scarring or stiffening. Uvulopalatopharyngoplasty was developed by Fujita in the 1980s but has significant associated morbidity and even mortality and as such, has fallen out of favour. Moreover, although the underlying theory is to augment the retropalatal dimension, success rates for OSAHS are low (73,74). Subsequently, further options have developed such as modified Z-palatoplasty and laser-assisted palatoplasty (75,76). Laser assisted palatoplasty has shown encouraging results in the short and long term and can be performed as a single staged procedure under general anaesthetic, ensuring a maximal excision of 25% of the length of the soft palate and 50% of the length of the uvula with concomitant redundant posterior pillar mucosa excision (Kotecha technique) (58,77,78). There has also been increasing interest in relocation and lateral pharynoglasty procedures. These surgeries focus on tissue repositioning, which can be extensive, rather than resection. In selected patients expansion sphincter pharyngoplasty (a type of lateral pharyngoplasty) and relocation pharyngoplasty have demonstrated promising results (79,80).
In the paediatric subgroup, adenotonsillectomy has conclusively been shown to improve OSAHS and improve long term quality of life (59,81,82). The clinical picture in adults is typically more complex and multifactorial however.
The contribution of the tongue base and epiglottis to snoring and OSAHS is underappreciated and underlines the relevance and importance of a dynamic visualisation of patient snoring cycles with DISE to assess for multilevel collapse (33). Surgery in this area can be quite challenging however. Minimally invasive options such as radiofrequency ablation to the tongue base have been shown to be efficacious (55,57,69,83,84). More aggressive procedures such as midline glossectomy and hyoid suspension have also been described with varying success rates (56,85,86). Hypoglossal nerve stimulation synchronised with inspiration via the surgical introduction of an electrical implant has shown recent promise with the underlying theory that reduced upper airway muscle activity is fundamental to OSAHS. Further research is required however as serious complications have been reported (87,88). The advent of the da Vinci system has led to the development of transoral robotic surgery to address hypopharyngeal collapse, with initial promising reports (89-91). Apart from tracheostomy, the highest success rates for snoring surgery have been achieved by maxillomandibular advancement (MMA) which increases retropalatal and retroglossal dimensions (92-94). Volumetric analysis confirms that MMA increases the air space of the pharynx by expanding the facial skeleton to which soft tissues of the pharynx and tongue are fixed, resulting in reduced collapsibility during the negative pressure of inspiration (92). MMA surgery is often neglected due to the perceived extent of the surgery and associated morbidity; however low complication rates have been reported in a recent meta-analysis, although patients do require a soft diet for two months and major complications can occur (95). Patient selection and consent is therefore once again of utmost importance.
Tracheostomy is rarely required but remains the definitive treatment for OSAHS as the upper airway is bypassed. Bariatric surgery has been of increasing interest and has been shown in cohort studies to improve OSAHS and sleep quality but would not be expected to ‘cure’ OSAHS or snoring (96). Combined treatment modalities, both non-surgical and surgical, will often be required in these patients and multilevel surgery is the norm. A multidisciplinary team is therefore required to manage these patients including the respiratory, orthodontic, maxillofacial and otolaryngology teams. In effect, patients with a dynamic physiological airway obstruction are being treated by a step wise anatomical ladder ranging from simple to more complex procedures. Management is individually tailored for patients depending on a multitude of factors and constant re-evaluation is necessary, for example with repeat sedation endoscopy following surgeries.
Conclusions
Snoring and OSAHS are increasingly common and involve a significant social and economic impact. Management requires a thorough clinical assessment, appropriate patient counselling and selection, and a multidisciplinary team. Both non-surgical and surgical options are efficacious in diligently selected patients. Surgery may be used either to facilitate CPAP usage or to bypass/improve anatomical obstructions. Hence, multilevel surgery is typically required. Further long term prospective studies are needed, with standardised data capture and measures of success, to produce a more robust evidence base.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89. [PubMed]
- AlGhanim N, Comondore VR, Fleetham J, et al. The economic impact of obstructive sleep apnea. Lung 2008;186:7-12. [PubMed]
- Foley D, Ancoli-Israel S, Britz P, et al. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res 2004;56:497-502. [PubMed]
- Namen AM, Dunagan DP, Fleischer A, et al. Increased physician-reported sleep apnea: the National Ambulatory Medical Care Survey. Chest 2002;121:1741-7. [PubMed]
- Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax 1991;46:85-90. [PubMed]
- Stradling JR, Crosby JH, Payne CD. Self reported snoring and daytime sleepiness in men aged 35-65 years. Thorax 1991;46:807-10. [PubMed]
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [PubMed]
- George CF, Smiley A. Sleep apnea & automobile crashes. Sleep 1999;22:790-5. [PubMed]
- He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest 1988;94:9-14. [PubMed]
- Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol 1983;52:490-4. [PubMed]
- Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034-41. [PubMed]
- Ip MS, Lam B, Ng MM, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 2002;165:670-6. [PubMed]
- Marshall NS, Wong KK, Liu PY, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31:1079-85. [PubMed]
- Schraufnagel DE. Breathing in America: Diseases, Progress, and Hope. USA: American Thoracic Society, 2010.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [PubMed]
- Kotecha B. The nose, snoring and obstructive sleep apnoea. Rhinology 2011;49:259-63. [PubMed]
- Virk JS, Nouraei R, Kotecha B. Multilevel radiofrequency ablation to the soft palate and tongue base: tips and pitfalls. Eur Arch Otorhinolaryngol 2014;271:1809-13. [PubMed]
- Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 1985;32:429-34. [PubMed]
- Friedman M, Hamilton C, Samuelson CG, et al. Diagnostic value of the Friedman tongue position and Mallampati classification for obstructive sleep apnea: a meta-analysis. Otolaryngol Head Neck Surg 2013;148:540-7. [PubMed]
- Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg 2002;127:13-21. [PubMed]
- Friedman M, Ibrahim H, Joseph NJ. Staging of obstructive sleep apnea/hypopnea syndrome: a guide to appropriate treatment. Laryngoscope 2004;114:454-9. [PubMed]
- Herzog M, Metz T, Schmidt A, et al. The prognostic value of simulated snoring in awake patients with suspected sleep-disordered breathing: introduction of a new technique of examination. Sleep 2006;29:1456-62. [PubMed]
- Ritter CT, Trudo FJ, Goldberg AN, et al. Quantitative evaluation of the upper airway during nasopharyngoscopy with the Müller maneuver. Laryngoscope 1999;109:954-63. [PubMed]
- Tunçel U, Inançli HM, Kürkçüoğlu SS, et al. Can the Müller maneuver detect multilevel obstruction of the upper airway in patients with obstructive sleep apnea syndrome? Kulak Burun Bogaz Ihtis Derg 2010;20:84-8. [PubMed]
- Health Quality Ontario. Polysomnography in patients with obstructive sleep apnea: an evidence-based analysis. Ont Health Technol Assess Ser 2006;6:1-38. [PubMed]
- Georgalas C, Garas G, Hadjihannas E, et al. Assessment of obstruction level and selection of patients for obstructive sleep apnoea surgery: an evidence-based approach. J Laryngol Otol 2010;124:1-9. [PubMed]
- Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci 1991;16:504-9. [PubMed]
- Abdullah VJ, Lee DL, Ha SC, et al. Sleep endoscopy with midazolam: sedation level evaluation with bispectral analysis. Otolaryngol Head Neck Surg 2013;148:331-7. [PubMed]
- Hewitt RJ, Dasgupta A, Singh A, et al. Is sleep nasendoscopy a valuable adjunct to clinical examination in the evaluation of upper airway obstruction? Eur Arch Otorhinolaryngol 2009;266:691-7. [PubMed]
- Kezirian EJ, White DP, Malhotra A, et al. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg 2010;136:393-7. [PubMed]
- Ravesloot MJ, de Vries N. One hundred consecutive patients undergoing drug-induced sleep endoscopy: results and evaluation. Laryngoscope 2011;121:2710-6. [PubMed]
- Chisholm E, Kotecha B. Oropharyngeal surgery for obstructive sleep apnoea in CPAP failures. Eur Arch Otorhinolaryngol 2007;264:51-5. [PubMed]
- Kotecha BT, Hannan SA, Khalil HM, et al. Sleep nasendoscopy: a 10-year retrospective audit study. Eur Arch Otorhinolaryngol 2007;264:1361-7. [PubMed]
- De Vito A, Carrasco Llatas M, Vanni A, et al. European position paper on drug-induced sedation endoscopy (DISE). Sleep Breath 2014;18:453-65. [PubMed]
- Patil SP. What every clinician should know about polysomnography. Respir Care 2010;55:1179-95. [PubMed]
- Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obstructive sleep apnea--a systematic review. Sleep 2009;32:27-36. [PubMed]
- Elshaug AG, Moss JR, Southcott AM, et al. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep 2007;30:461-7. [PubMed]
- Lin HC, Friedman M, Chang HW, et al. The efficacy of multilevel surgery of the upper airway in adults with obstructive sleep apnea/hypopnea syndrome. Laryngoscope 2008;118:902-8. [PubMed]
- Field CJ, Robinson S, Mackay S, et al. Clinical equipoise in sleep surgery: investigating clinical trial targets. Otolaryngol Head Neck Surg 2011;145:347-53. [PubMed]
- Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev 2005.CD001004. [PubMed]
- Ha SC, Hirai HW, Tsoi KK. Comparison of positional therapy versus continuous positive airway pressure in patients with positional obstructive sleep apnea: a meta-analysis of randomized trials. Sleep Med Rev 2014;18:19-24. [PubMed]
- Oksenberg A, Gadoth N. Are we missing a simple treatment for most adult sleep apnea patients? The avoidance of the supine sleep position. J Sleep Res 2014;23:204-10. [PubMed]
- van Maanen JP, Meester KA, Dun LN, et al. The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath 2013;17:771-9. [PubMed]
- van Maanen JP, Richard W, Van Kesteren ER, et al. Evaluation of a new simple treatment for positional sleep apnoea patients. J Sleep Res 2012;21:322-9. [PubMed]
- Johansson K, Hemmingsson E, Harlid R, et al. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ 2011;342:d3017. [PubMed]
- Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med 2010;6:238-43. [PubMed]
- Johal A, Battagel JM, Kotecha BT. Sleep nasendoscopy: a diagnostic tool for predicting treatment success with mandibular advancement splints in obstructive sleep apnoea. Eur J Orthod 2005;27:607-14. [PubMed]
- Battagel JM, Johal A, Kotecha BT. Sleep nasendoscopy as a predictor of treatment success in snorers using mandibular advancement splints. J Laryngol Otol 2005;119:106-12. [PubMed]
- Johal A, Arya D, Winchester LJ, et al. The effect of a mandibular advancement splint in subjects with sleep-related breathing disorders. Br Dent J 2005;199:591-6; discussion 581; quiz 608. [PubMed]
- Johal A, Gill G, Ferman A, et al. The effect of mandibular advancement appliances on awake upper airway and masticatory muscle activity in patients with obstructive sleep apnoea. Clin Physiol Funct Imaging 2007;27:47-53. [PubMed]
- Johal A, Hector MP, Battagel JM, et al. Impact of sleep nasendoscopy on the outcome of mandibular advancement splint therapy in subjects with sleep-related breathing disorders. J Laryngol Otol 2007;121:668-75. [PubMed]
- Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest 2007;132:1057-72. [PubMed]
- NICE. Continuous positive airway pressure for obstructive sleep pnoea/hypopnoea syndrome. Available online: https://www.nice.org.uk/guidance/ta139/resources/continuous-positive-airway-pressure-for-obstructive-sleep-apnoeahypopnoea-syndrome-374791501
- Elshaug AG, Moss JR, Hiller JE, et al. Upper airway surgery should not be first line treatment for obstructive sleep apnoea in adults. BMJ 2008;336:44-5. [PubMed]
- Lim DJ, Kang SH, Kim BH, et al. Treatment of obstructive sleep apnea syndrome using radiofrequency-assisted uvulopalatoplasty with tonsillectomy. Eur Arch Otorhinolaryngol 2013;270:585-93. [PubMed]
- Bowden MT, Kezirian EJ, Utley D, et al. Outcomes of hyoid suspension for the treatment of obstructive sleep apnea. Arch Otolaryngol Head Neck Surg 2005;131:440-5. [PubMed]
- De Vito A, Frassineti S, Panatta ML, et al. Multilevel radiofrequency ablation for snoring and OSAHS patients therapy: long-term outcomes. Eur Arch Otorhinolaryngol 2012;269:321-30. [PubMed]
- Iyngkaran T, Kanagalingam J, Rajeswaran R, et al. Long-term outcomes of laser-assisted uvulopalatoplasty in 168 patients with snoring. J Laryngol Otol 2006;120:932-8. [PubMed]
- Randhawa PS, Cetto R, Chilvers G, et al. Long-term quality-of-life outcomes in children undergoing adenotonsillectomy for obstructive sleep apnoea: a longitudinal study. Clin Otolaryngol 2011;36:475-81. [PubMed]
- Hultcrantz E, Harder L, Loord H, et al. Long-term effects of radiofrequency ablation of the soft palate on snoring. Eur Arch Otorhinolaryngol 2010;267:137-42. [PubMed]
- Thatcher GW, Maisel RH. The long-term evaluation of tracheostomy in the management of severe obstructive sleep apnea. Laryngoscope 2003;113:201-4. [PubMed]
- Berry S, Roblin G, Williams A, et al. Validity of sleep nasendoscopy in the investigation of sleep related breathing disorders. Laryngoscope 2005;115:538-40. [PubMed]
- Li HY, Lee LA, Wang PC, et al. Nasal surgery for snoring in patients with obstructive sleep apnea. Laryngoscope 2008;118:354-9. [PubMed]
- Verse T, Maurer JT, Pirsig W. Effect of nasal surgery on sleep-related breathing disorders. Laryngoscope 2002;112:64-8. [PubMed]
- Powell NB, Zonato AI, Weaver EM, et al. Radiofrequency treatment of turbinate hypertrophy in subjects using continuous positive airway pressure: a randomized, double-blind, placebo-controlled clinical pilot trial. Laryngoscope 2001;111:1783-90. [PubMed]
- Brietzke SE, Mair EA. Injection snoreplasty: how to treat snoring without all the pain and expense. Otolaryngol Head Neck Surg 2001;124:503-10. [PubMed]
- Ho WK, Wei WI, Chung KF. Managing disturbing snoring with palatal implants: a pilot study. Arch Otolaryngol Head Neck Surg 2004;130:753-8. [PubMed]
- NICE. Interventional procedure overview of radiofrequency ablation of the soft palate for snoring. Available online: https://www.nice.org.uk/guidance/ipg476/documents/radiofrequency-ablation-of-the-soft-palate-for-snoring-overview2
- Farrar J, Ryan J, Oliver E, et al. Radiofrequency ablation for the treatment of obstructive sleep apnea: a meta-analysis. Laryngoscope 2008;118:1878-83. [PubMed]
- Bäck LJ, Hytönen ML, Roine RP, et al. Radiofrequency ablation treatment of soft palate for patients with snoring: a systematic review of effectiveness and adverse effects. Laryngoscope 2009;119:1241-50. [PubMed]
- Stuck BA. Radiofrequency-assisted uvulopalatoplasty for snoring: Long-term follow-up. Laryngoscope 2009;119:1617-20. [PubMed]
- Bäck LJ, Liukko T, Sinkkonen ST, et al. Complication rates of radiofrequency surgery in the upper airways: a single institution experience. Acta Otolaryngol 2009;129:1469-73. [PubMed]
- Fujita S, Conway W, Zorick F, et al. Surgical correction of anatomic azbnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981;89:923-34. [PubMed]
- Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996;19:156-77. [PubMed]
- Lin HC, Friedman M, Chang HW, et al. Z-palatopharyngoplasty plus radiofrequency tongue base reduction for moderate/severe obstructive sleep apnea/hypopnea syndrome. Acta Otolaryngol 2010;130:1070-6. [PubMed]
- Kamami YV. Laser CO2 for snoring. Preliminary results. Acta Otorhinolaryngol Belg 1990;44:451-6. [PubMed]
- Kotecha B, Paun S, Leong P, et al. Laser Assisted Uvulopalatoplasty: an objective evaluation of the technique and results. Clin Otolaryngol Allied Sci 1998;23:354-9. [PubMed]
- Patel N, Gill J, Kotecha B. How I do it--the Kotecha technique for laser palatoplasty. Eur Arch Otorhinolaryngol 2006;263:152-5. [PubMed]
- Pang KP, Woodson BT. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg 2007;137:110-4. [PubMed]
- Li HY, Lee LA. Relocation pharyngoplasty for obstructive sleep apnea. Laryngoscope 2009;119:2472-7. [PubMed]
- Swift AC. Upper airway obstruction, sleep disturbance and adenotonsillectomy in children. J Laryngol Otol 1988;102:419-22. [PubMed]
- Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med 2013;368:2366-76. [PubMed]
- Fischer Y, Khan M, Mann WJ. Multilevel temperature-controlled radiofrequency therapy of soft palate, base of tongue, and tonsils in adults with obstructive sleep apnea. Laryngoscope 2003;113:1786-91. [PubMed]
- Simunjak B, Slipac J, Krmpotić P, et al. Efficiency of radiofrequency assisted uvulopalatopharyngoplasty in the treatment of snoring. Acta Clin Croat 2011;50:357-60. [PubMed]
- Chabolle F, Wagner I, Blumen MB, et al. Tongue base reduction with hyoepiglottoplasty: a treatment for severe obstructive sleep apnea. Laryngoscope 1999;109:1273-80. [PubMed]
- Suh GD. Evaluation of open midline glossectomy in the multilevel surgical management of obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 2013;148:166-71. [PubMed]
- Eastwood PR, Barnes M, Walsh JH, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep 2011;34:1479-86. [PubMed]
- Kezirian EJ, Boudewyns A, Eisele DW, et al. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev 2010;14:299-305. [PubMed]
- Friedman M, Hamilton C, Samuelson CG, et al. Transoral robotic glossectomy for the treatment of obstructive sleep apnea-hypopnea syndrome. Otolaryngol Head Neck Surg 2012;146:854-62. [PubMed]
- Lin HS, Rowley JA, Badr MS, et al. Transoral robotic surgery for treatment of obstructive sleep apnea-hypopnea syndrome. Laryngoscope 2013;123:1811-6. [PubMed]
- Vicini C, Dallan I, Canzi P, et al. Transoral robotic surgery of the tongue base in obstructive sleep Apnea-Hypopnea syndrome: anatomic considerations and clinical experience. Head Neck 2012;34:15-22. [PubMed]
- Faria AC, da Silva-Junior SN, Garcia LV, et al. Volumetric analysis of the pharynx in patients with obstructive sleep apnea (OSA) treated with maxillomandibular advancement (MMA). Sleep Breath 2013;17:395-401. [PubMed]
- Faria AC, Xavier SP, Silva SN Jr, et al. Cephalometric analysis of modifications of the pharynx due to maxillo-mandibular advancement surgery in patients with obstructive sleep apnea. Int J Oral Maxillofac Surg 2013;42:579-84. [PubMed]
- Hsieh YJ, Liao YF. Effects of maxillomandibular advancement on the upper airway and surrounding structures in patients with obstructive sleep apnoea: a systematic review. Br J Oral Maxillofac Surg 2013;51:834-40. [PubMed]
- Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 2010;14:287-97. [PubMed]
- Haines KL, Nelson LG, Gonzalez R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery 2007;141:354-8. [PubMed]


