Clinical pathway for thoracic surgery in an Italian centre
Introduction
Clinical care pathways, formally known as physician directed diagnostic and therapeutic plans (1). The main driving force to develop clinical pathway of care is to improve quality of care but due to the tremendous pressure to reduce costs, clinical pathway of care has been embraced as a mean to achieve both goals (2). In literature several papers demonstrated that the application of clinical pathways to patients undergoing pulmonary resections realized a significant reduction in hospital charges, improving quality and with acceptable hospital readmission rate (2-5). Creation and maintenance of clinical pathways of care at our Thoracic Surgery Unit (Ospedali Riuniti, Ancona, Italy) has been a continuous evolving process. We did every effort to apply and follow evidence based approaches when possible in order to improve quality.
We report the clinical pathway of care adopted at our centre since the recent introduction of Uniportal VATS program for major lung resections (lobectomies/segmentectomies).
Patients and methods
This is a prospectively collected data of 102 consecutive patients submitted to pulmonary lobectomy [83] or segmentectomy [19] from March 2014 till September 2015. Table 1 shows patients baseline characteristics.
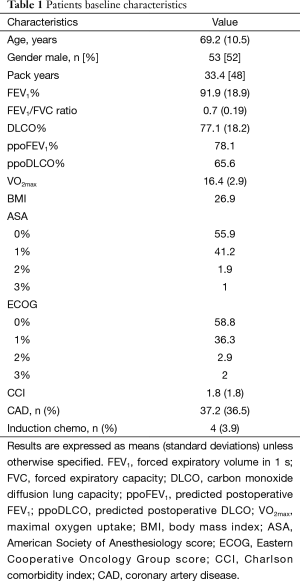
Full table
All operations were performed or supervised by a single qualified thoracic surgeon (Dr. Majed Refai).
Patients with a predicted postoperative forced expiratory volume in 1 s and predicted postoperative carbon monoxide lung diffusing capacity <30% and VO2 peak 6,7) were excluded from anatomical lobar or sublobar resections.
Surgical technique
The patient is placed in a lateral decubitus position. A single 4-cm incision is performed in the 5th intercostal space with no rib spreading. We use wound protector (Alexis wound protector, Applied Medical, USA). The camera (a 5-mm 30°) is introduced in the same incision with no trocar. In complete fissures were developed by using stapling devices. The bronchus and vessels were closed and cut by mechanical staplers. Recently we began using vascular clips (Hem-o-lok ligation system, Teleflex, USA) to close small vessels less than or equal to 5 mm.
In case of segmentectomy the operation is done through a single 4-cm incision as for lobectomy, vessels (arteries and veins) are identified and stapled. Once the bronchus is closed, the parenchymal plane is identified by inflation and then divided by stapling.
Systematic lymph node dissection is performed in all patients.
In both procedures, once the operation is completed, we insert a single chest tube Ch24 in the posterior part of the incision.
Intraoperative and postoperative standardized protocols were implemented aimed at fast tracking patients submitted to major lung resections [Uniportal (video-assisted thoracic surgery) VATS lobectomy/segmentectomy].
Intraoperative protocols
Operative technique
Fissureless technique is used for upper lobectomies and with a systematic use for right upper lobectomy (8). For lower lobectomies with no artery visible in the fissure and with incomplete fissure, fissureless technique is performed.
Air leak assessment
Objective assessment of air leak (with ventilator spirometer), expressed as mL/min, was performed before closure of the chest by measuring the difference between a fixed inspired and expired volume using a tidal volume of 8 mL/kg, respiratory rate of 10 and a peak-end-expiratory pressure of 5 cmH2O.
Check the cause of air leak the bronchus or pulmonary parenchyma.
Correction to reduce air leak was performed by applying stitches or by the use of staples.
Single chest tube
We use a single 24-French chest tube (Redax, PVC radiopaque chest tube) placed in the posteriore part of the incision (9,10).
Intraoperative pain control
We infiltrate three intercostal spaces (IV, V and VI intercostal spaces) with ropivacaina 0.75% at the end of the operation under thoracoscopic vision (10). No epidural catheter is inserted to facilitate early mobilization.
As a rule patients were extubated in the operating room and transferred to a dedicated general thoracic surgery ward. Planned admission to intensive care was reserved for patients with life-threatening cardiopulmonary complications needing active life-support treatments.
Postoperative protocols
Implementation of standardized treatment protocols for the most frequent complications after major lung resections are atrial fibrillation, pneumonia and atelectasis (11).
Atrial fibrillation
Atrial fibrillation is described as a considerable complication even after minimally invasive thoracic procedures with a rate ranging from 4% to 12% after thoracoscopic lobectomy (12). So patient monitoring is needed and more attention must be paid for patients with a history of cardiac arrhythmias.
On operation day the patient’s electrocardiogram, heart rate, pulse oximetry and respiratory rate are monitored continuously.
On preoperative day (POD) 1, the same parameters are verified every 3 hours and the electrocardiogram is performed in any suspected disorders of heart rhythm.
If atrial fibrillation is detected we adopt the following strategies (13):
- Patient hemodynamically stable with cardiac history negative for coronary artery disease, heart failure, rhythm disorders:
- Patient at rest;
- Check and correct, whenever possible, any potential factor able to trigger or maintain the arrhythmia (electrolytic disorders, hypoxemia..);
- Begin medical treatment;
- Perform cardiologic evaluation in the case where the heart rhythm is not restored;
- Patient hemodynamically not stable or cardiac history positive for coronary artery disease, heart failure, rhythm disorders:
- Patient at rest;
- Cardiologic evaluation for the ideal pharmacological treatment or electrical cardioversion.
In both cases, we reintroduce a continuous electrocardiographic and heart rate monitoring.
Pneumonia
The diagnosis of a postoperative pneumonia is obtained following strictly the criteria proposed by the CDC and more recently adopted by the European Society of Thoracic Surgeons (ESTS) and The Society of Thoracic Surgeons (STS) (14). The pneumonia represents the most frequent pulmonary complication following VATS lobectomy, with a rate around 3% in a large series from the STS database (15).
So, in any case of symptoms suggestive of pneumonia [fever, leukocytosis >12,000 white blood cells (WBC)/mm3, purulent sputum, worsening cough, dyspnea, rales, desaturation], we perform a chest radiograph in order to definitively confirm the diagnosis.
If the chest X-ray shows a lung infiltrate or a consolidation:
- Try to obtain a lower respiratory tract culture;
- Start appropriate, broad-spectrum, antibiotic therapy;
- Intensify respiratory nebulisers;
- Begin a continuous pulse oximeter monitoring;
- Check the temperature every 4 hours;
- Monitor the WBC count and the C-reactive protein (CRP).
In absence of clinical improvement after 48–72 hours:
(I) Adjust the antibiotic therapy if the culture is positive;
(II) Search for other pathogens or other sites of infections if the culture is negative infectologist evaluation.
Atelectasis
Despite the lesser impact on the chest wall and a more gentle manipulation of the lung during the operation, the postoperative atelectasis rate after VATS lobectomy is not significantly lower, albeit reduced, in comparison to the one registered after the open thoracotomy approach (VATS lobectomy: 2.1% vs. open lobectomy: 3.3%) (15).
In the case of new-onset dyspnea or desaturation, we always perform a chest radiograph.
If the chest X-ray confirms an atelectasis:
- Intensify respiratory nebulisers;
- Intensify chest physiotherapy (16);
- Perform a bronchoscopy to clean the endobronchial secretions (17);
- Begin a continuous pulse oximeter monitoring;
- Take a post-bronchoscopy chest radiograph to monitor the lung expansion improvement.
Clinical protocols for chest tube management
Standardized protocol for early chest tube removal after major lung resection
Use of electronic drainage systems for the objective measurement of postoperative air-leak and the adoption of 400 mL/day of pleural effusion for chest tube removal is used as standardized protocol for early chest tube removal after major lung resection.
We chose to use an electronic chest drainage system (Thopaz, Medela Healthcare, Switzerland) capable of varying the suction level according to the feedback received from the pleural cavity (variable suction) in order to maintain a stable pressure corresponding to the preset value. This device appears ideal to study the effect of different levels of pressure on the duration of air leak (18).
The preset pressure is −8 cmH2O on the operation day, the device works passively and we can monitor and record the pleural pressure driven by the patient himself. Chest tube is removed when air leak stops and the pressure curve raises to physiologic range. The pressure is stabilized by the patient himself.
Chest physiotherapy
Ward nurses encourage patients for early mobilization and deep breathing by the use of incentive spirometer on POD1.
Dedicated chest physiotherapist begins the treatment program on the POD1, and follows the patient till discharge.
Patients with pneumonia, atelectasis or both were treated by the chest physiotherapist twice a day.
All patients were asked to repeat the exercises learned in the morning more than once a day.
Chest physiotherapy performed by specialized therapists may produce functional benefits in resectable lung cancer patients and is recommended (6).
Patient counselling
Pre-emptive explanation and planning of the fast-tracking protocol to the patient before the admission and daily updating after the operation.
The plan for discharge was discussed with patients and families during rounds on POD2.
Postoperative daily protocol for patients who undergoes uniportal VATS lobectomy/segmentectomy was designed (Table 2).
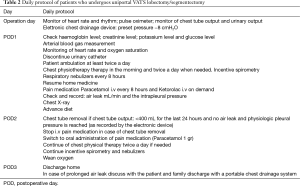
Full table
It was applied to 102 consecutive patients submitted to uniportal VATS lobectomy or uniportal VATS segmentectomy.
Twice-daily consultant ward rounds with dedicated thoracic nurse.
Patient discharge was discussed with patients and families on POD3.
In case of prolonged air leak the patients were discharged with a portable chest drainage device.
Early outpatient follow-up
All patients underwent ambulatory visit 10 days after discharge: chest X-ray was performed, clinical control and removal of stitches.
Results
There were 102 patients, with a median age of 69 years. Types of pulmonary resections performed were 83 uniportal VATS lobectomies (81%) and 19 uniportal VATS segmentectomies (19%) (Table 1).
A total of 38 (37%) patients had pre-existing coronary artery disease (history of coronary artery bypass or angioplasty), 3 (2.9%) patients had congestive heart failure.
All patients were extubated in the operating room. Only one patient went to the ICU (1%) due to previous history of a recent pulmonary emboli and coronary artery disease (coronary artery bypass) (1 day stay) (Table 3).
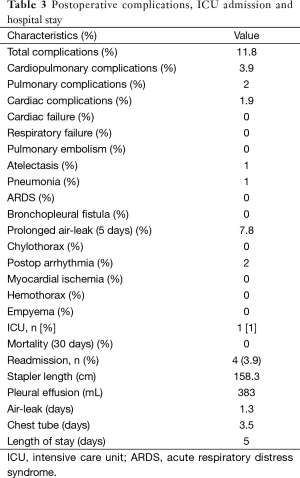
Full table
No mortality occurred (within the hospital stay and 30 days from discharge).
Pulmonary complications (2%): we had one pneumonia and one atelectasis that necessitate fibrobroncoscopy.
Cardiac complications (2%): two postoperative atrial fibrillation where sinus rhythm was restored by amiodarone.
Eight patients had prolonged air leak (more than 5 days) (7.8%).
The median pleural effusion within the first 48 hours was 383 mL.
Chest tube days are 3.5 days and the median hospital stay was 5 days.
Only four patients were readmitted (3.9%) within 30 days of discharge (Table 3). The main reason for readmission was pneumothorax (3 pts) that necessitated chest drain and pneumonia (1 pt) treated with antibiotics, expectorants, respiratory nebulisers and chest physiotherapy.
Comment
Clinical pathways of care are essential to the introduction of evidence based medicine and clinical guidelines in our daily practice.
In our unit at Ospedali Riuniti, Ancona, clinical pathway of care was an evolving process. We changed our protocols with the introduction of new technology and new surgical techniques.
Clinical pathways of care implementation can be challenging. It should include all staff members’ education and concerns, and misconceptions about the pathway development should be addressed. Data must be collected and analysed and the process must be improved to achieve the goals of resource savings with improvement in outcome (11).
The most important tool for any quality assessment is a database that should be prospectively maintained and periodically audited (19).
We report our clinical pathway of care applied to consecutive 102 uniportal VATS (lobectomy/segmentectomy).
A refinement of both intraoperative procedures and perioperative strategies has led to a reduction of the postoperative hospital stay and improved quality.
Our pulmonary complications (2%) and cardiac complications (2%) were low.
Our readmission rate was low (3.9%). Rajaram et al., reported readmission rate of (8.4%) after vats lobectomy (20). The authors suggested early outpatient follow-up and more attention to reduce postoperative complications.
At our centre early outpatient follow-up was adopted. All patients underwent an ambulatory visit, performed by a thoracic surgery consultant, 10 days after discharge. Clinical control and chest X-ray was performed.
In summary
In order to integrate a clinical pathway in thoracic surgery you need:
- A database prospectively maintained and periodically audited;
- Statement of goals and key elements of care based on evidence and best practice;
- Communication among all stuff members and with patients and families;
- Identification of the appropriate resources.
Acknowledgements
To Francesca Manzotti our physiotherapist and to her colleagues who collaborate with us. To all Nurses of the division of Thoracic surgery and to all Nurses of the operating room.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hart R, Musfeldt C. MD-directed critical pathways: it's time. Hospitals 1992;66:56. [PubMed]
- Zehr KJ, Dawson PB, Yang SC, et al. Standardized clinical care pathways for major thoracic cases reduce hospital costs. Ann Thorac Surg 1998;66:914-9. [PubMed]
- Wright CD, Wain JC, Grillo HC, et al. Pulmonary lobectomy patient care pathway: a model to control cost and maintain quality. Ann Thorac Surg 1997;64:299-302. [PubMed]
- Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. [PubMed]
- Salati M, Brunelli A, Xiumè F, et al. Does fast-tracking increase the readmission rate after pulmonary resection? A case-matched study. Eur J Cardiothorac Surg 2012;41:1083-7; discussion 1087. [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Refai M, Brunelli A, Salati M, et al. Efficacy of anterior fissureless technique for right upper lobectomies: a case-matched analysis. Eur J Cardiothorac Surg 2011;39:1043-6. [PubMed]
- Okur E, Baysungur V, Tezel C, et al. Comparison of the single or double chest tube applications after pulmonary lobectomies. Eur J Cardiothorac Surg 2009;35:32-5; discussion 35-6. [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [PubMed]
- Every NR, Hochman J, Becker R, et al. Critical pathways: a review. Committee on Acute Cardiac Care, Council on Clinical Cardiology, American Heart Association. Circulation 2000;101:461-5. [PubMed]
- Park BJ, Zhang H, Rusch VW, et al. Video-assisted thoracic surgery does not reduce the incidence of postoperative atrial fibrillation after pulmonary lobectomy. J Thorac Cardiovasc Surg 2007;133:775-9. [PubMed]
- Fernando HC, Jaklitsch MT, Walsh GL, et al. The Society of Thoracic Surgeons practice guideline on the prophylaxis and management of atrial fibrillation associated with general thoracic surgery: executive summary. Ann Thorac Surg 2011;92:1144-52. [PubMed]
- American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [PubMed]
- McCool FD, Rosen MJ. Nonpharmacologic airway clearance therapies: ACCP evidence-based clinical practice guidelines. Chest 2006;129:250S-259S. [PubMed]
- Korst RJ, Humphrey CB. Complete lobar collapse following pulmonary lobectomy. Its incidence, predisposing factors, and clinical ramifications. Chest 1997;111:1285-9. [PubMed]
- Brunelli A, Salati M, Pompili C, et al. Regulated tailored suction vs regulated seal: a prospective randomized trial on air leak duration. Eur J Cardiothorac Surg 2013;43:899-904. [PubMed]
- Brunelli A, Varela G, Berrisford R, et al. Audit, quality control, and performance in thoracic surgery--a European perspective. Thorac Surg Clin 2007;17:387-93. vii. [PubMed]
- Rajaram R, Ju MH, Bilimoria KY, et al. National evaluation of hospital readmission after pulmonary resection. J Thorac Cardiovasc Surg 2015;150:1508-1514.e2.


