Left- and right-sided video-assisted thoracoscopic thymectomy exhibit similar effects on myasthenia gravis
Introduction
Despite the lack of evidence-based medicine, thymectomy has been widely used for treatment of patients with myasthenia gravis (MG) for nearly a century. The effectiveness of thymectomy and medication alone is difficult to compare in a randomized controlled study because of the low prevalence of MG, medication requirement after surgery, and long time required to achieve remission. Nevertheless, several retrospective studies suggest a positive correlation between thymectomy and prognosis of MG (1,2). A meta-analysis conducted by the American Academy of Neurology illustrated the superiority of surgical treatments over non-surgical treatments regardless of the differences in selection criteria and outcome assessment (2). Therefore, thymectomy is the generally recommended option to achieve MG remission or improvement.
The surgical approach of thymectomy can be classified into transsternal (TS), transcervical, and video-assisted thoracoscopic (VATS) surgery. TS extended thymectomy has been considered the standard surgical approach, despite that this technique causes long-lasting postoperative pain and cutaneous scar, which may lead to the delay of surgical treatment. Transcervical thymectomy minimizes surgical trauma and improves outcome acceptance, particularly for young women who occupy a large proportion of patients with MG. Nevertheless, the remission rate of transcervical thymectomy may be less satisfied because it exhibits higher probability of leaving ectopic thymic tissue (3). VATS thymectomy was first reported by Sugarbaker in 1993 (4), and has been rapidly developed since then. This technique presents evident advantages, particularly shorter operative time, less operative trauma, improved cosmesis, and shorter hospital stay but similar efficiency to TS thymectomy (5,6). VATS thymectomy can be categorized as unilateral (right or left), bilateral, subxiphoid, and bilateral VATS with cervical incision. Resection potential and treatment outcomes have been investigated. Bilateral VATS thymectomy with and without cervical incision were speculated to be more thorough than unilateral VATS thymectomy because of better visualization of both sides of the mediastinum and cervical thymic lobes; nevertheless, these techniques exhibit similar therapeutic effects (7). Unilateral VATS, particularly the procedure performed on the right side, is clinically preferred; in this technique, the landmark of the superior vena cava, where the left innominate vein converges, can be conveniently identified (8). However, other researchers recommended left-sided VATS thymectomy (9). Hence, advantages, securities, and difficulties must be compared between unilateral VATS thymectomy performed on each side of patients with MG.
This study presents our experience regarding VATS thymectomy, compares short-term outcomes with that of TS procedures, and identifies differences of unilateral VATS thymectomy conducted on both sides.
Patients and methods
Study design and patients
This study was permitted by the Ethics Committee of Sun Yat-sen Memorial Hospital. Informed consents were provided by all of the participants. From January 2003 to December 2012, data of 81 consecutive patients with MG who underwent extended thymectomy at the Department of Thoracic Surgery Sun Yat-sen Memorial Hospital were retrospectively reviewed. All the patients were diagnosed with MG based on the weakness and fatigability of affected muscles, decremental responses to repetitive nerve stimulation tests, and striking responses to intramuscular injection of one bolus of neostigmine methyl sulfate. A team consisting of experienced neurologists and thoracic surgeons worked to treat the patients. The neurologists were responsible for the diagnosis, classification, pharmaceutical treatment, efficacy assessment, and follow-up of the patients, whereas the thoracic surgeons took charge of the surgery and perioperative care.
All the patients are taking anticholinesterase agent till the day before surgery. A single stress dose of a corticosteroid was given before induction of anesthesia. Inhaled anesthetic agents provide sufficient muscle relaxation. Neuromuscular blocking agents are avoided. Postoperatively, patients are extubated in the operating room. The same dose of anticholnesterase as preoperatively was prescribed to them to control the MG symptom.
Clinicopathologic data, including gender, age at disease onset, date of surgery, clinical classification, medication, surgery information, morbidity, thymoma histology, maximum diameter, and position were obtained from clinical and pathologic records. Each patient with thymoma was staged according to Masaoka staging system (10). After the surgery, all the patients were followed up and drug doses were adjusted according to variations in MG symptoms. The patients were required to visit the neurologists at 2-month intervals if their symptoms persisted or at 6-month intervals if they were symptom free. Preoperative disease status was classified based on the Myasthenia Gravis Foundation of America (MGFA) classification (11). Postoperative responses after thymectomy were classified according to the modified post-intervention status scoring system of the MGFA (12); these responses were assessed using the following grading scale: Grade 1 indicates a complete stable remission without medications for at least 2 years, Grade 2 implies an asymptomatic status with pharmacological remission, Grade 3 signifies minimal manifestation of decreased pretreatment clinical symptoms or MG medications, Grade 4 reflects no change, and Grade 5 signifies worsening symptoms.
Operative procedures
Extended thymectomy was performed on all of the patients through unilateral VATS or TS resection. All the surgical procedures were performed by qualified thoracic surgeons during the study period. TS resection was initially the main procedure in the treatment of patients with MG. VATS has been conducted since 2006 through right- or left-sided thoracoscopic approach and is primarily determined by thymoma location, as well as surgeon’s preference and experience. Thymomas larger than 8 cm or with suspected vascular invasion were resected through the TS approach. The surgical techniques employed are described in previous studies (13,14).
Similar right- and left-sided VATS were performed. The pleura overlying the thymus is incised along the phrenic nerve. The thymus is then dissected form the anterior pericardium and followed upward to the neck. Dissection is continued deep inside, incising the contralateral pleura to visualize the phrenic nerve in the other hemithorax. Visualization of the innominate vein and its branches may be the only difference between the two procedures. In the right-sided VATS, the innominate vein can be easily separated when dissecting along the superior vena cava. However, the typical organ is absent in dividing innominate vein in the left-sided VATS.
Statistical analysis
All statistical analyses were performed with the SPSS 16.0 program package (SPSS, Inc. Chicago, IL USA). Continuous data were compared using Student’s t-test, and categorical variables were assessed using the Pearson Chi-square test or Fisher’s exact test as appropriate. Complete stable remissions (CSR) during the follow-up period were calculated by the Kaplan-Meier method, and compared by the log-rank tests. Multivariate analysis was performed using the logistic regression model with potential factors whose P values were less than 0.10 in the univariate analysis. Result was considered statistically significant when the two-tailed P value was less than or equal to 0.05.
Results
Patient characteristics
Eighty-one consecutive patients with MG (33 males and 48 females) were enrolled in the study. The average age of the patients at thymectomy was 35.5 years (range, 8–73 years). TS thymectomy was conducted on 50 (61.7%) patients, whereas unilateral VATS approaches were performed on the remaining 31 (38.3%) patients, 15 on the left side and 16 on the right side. No conversion from VATS to TS thymectomy was implemented. The MGFA clinical classification ranged from class I to class V. Ocular MG (class I) was the most common class and observed in 48 patients (59.3%). Before thymectomy, 77 patients (95.1%) required pyridostigmine bromide to relieve symptoms, 58 patients (71.6%) were given with prednisone, and 12 patients (14.8%) received intravenous immunoglobulin (IVIG) therapy. After the surgery, patient development was followed up for at least 2 years. The median length of follow-up for the overall population was 76.0 months (range, 25–166 months). After the surgery, pyridostigmine bromide and prednisone were successfully discontinued in 33 (40.7%) and 31 (38.3%) patients, respectively. IVIG treatment was no longer needed in any of these patients.
VATS versus transsternal (TS) thymectomy
Gender, age at onset, symptom duration, MGFA classification, and histology were not statistically significantly different between the VATS and TS groups. The follow-up period was significantly shorter in the VATS group because the VATS approach has not been adopted in our practice until 2006 (Table 1).
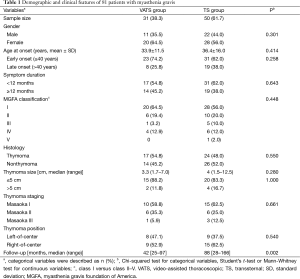
Full table
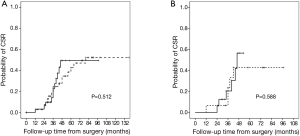
The perioperative outcomes of the 81 patients in the VATS and TS groups are listed in Table 2. The VATS group exhibited a significantly shorter surgery duration (P<0.001), less intraoperative blood loss (P=0.009), shorter postoperative hospital stay (P=0.025), smaller thoracic drainage volume (P=0.033), shorter thoracic drainage duration (P=0.006), and less postoperative complications (P<0.001) than the TS group. The most common postoperative complications included MG crisis (P=0.025) and arrhythmia (P=0.002), whose incidences were significantly reduced in the VATS group. Nevertheless, disease remission rate was not statistically different between the TS and VATS groups (P=0.988). The 5-year CSR in VATS and TS groups were 52.1% and 42%, respectively. The difference between them was not statistically significant (P=0.512, Figure 1A).
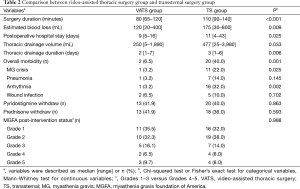
Full table
Left-sided versus right-sided VATS thymectomy
Baseline characteristics, including gender, age at onset, and MGFA classification, were not significantly different between the left- and right-sided groups. Furthermore, surgery duration, intraoperative blood loss, postoperative hospital stay, thoracic drainage volume, and postoperative complications were not significantly different. The time for thoracic drainage was significantly shorter in the right-sided group (P=0.041). Disease remission rate was not significantly different between the left- and right-sided groups (P=1.000). The 5-year CSR in left- and right-sided groups were 42.7% and 64.3%, respectively. The difference between them was not statistically significant (P=0.588, Figure 1B). Seventeen patients were preoperatively suspected with thymoma, which was confirmed after pathological diagnoses. Although more left-of-center thymomas were found in the left-sided group, the difference between the groups was not significant (Table 3).
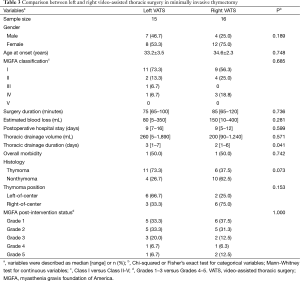
Full table
Favorable factors of disease remission
According to the MGFA post-intervention status, 68 patients in Grades 1–3 were considered in clinical remission. The 2-year clinical remission and complete remission rates were 84.0% (68/81) and 33.3% (27/81), respectively. In the univariate analysis, only the ocular type of MG was significantly found to be a favorable predicator for clinical remission (P=0.005). Surgical approach was not associated with remission rates (Table 4). In the analysis of the multivariate model after adjusting for gender, age at onset, symptom duration, histology, and surgical approach, the ocular type of MG remained as significant independent factor (odd ratio: 6.522, 95% confidence interval: 1.633–26.042, P=0.002).
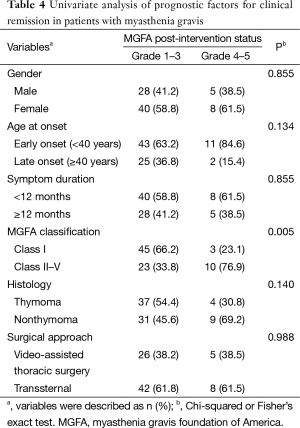
Full table
Discussion
As a minimally invasive option for the treatment of MG, VATS plays an increasingly role in therapy for MG, and has been one of the most common surgical approaches, because of its minimal invasiveness. Some researchers reported the possibility of incomplete resection because of visual limitation in VATS, which might be the key to MG healing (15). However, studies reported that successful symptom improvement did not differ between TS and VATS thymectomy (5,14). Data in our study also indicated that the VATS group exhibited a shorter surgical time, less intraoperative blood loss, less postoperative hospital stay, less thoracic drainage, and less postoperative complications but the similar remission rates to the TS group. Rückert et al. (16) demonstrated improved preserved pulmonary function in the immediate postoperative period after VATS thymectomy; this condition leads to less pulmonary infection and fast recovery. Chicaiza-Becerra et al. (17) reported that VATS thymectomy is a cost-effective strategy in treatment of patients in developing countries. These findings consolidated the position of VATS thymectomy in treatment of patients with MG, although a randomized, prospective clinical investigation must be further performed.
The side that is the better route of VATS remains controversial because thymus is often located in the middle of the mediastinum. In 1995, Yim et al. (18) proposed that a right-sided approach could be appropriate for VATS. The main advantages of the right-sided approach include larger operating space for the scope and equipment in the right pleura cavity and easily recognizable innominate vein. However, Mineo et al. (9) preferred the left-sided pathway because the left side of the thymus appears usually larger extending down to the pericardiophrenic area; this approach enables an extensive removal of fat allocated in the aortocaval groove, aortopulmonary window, and both pericardiophrenic sides. An anatomic study also demonstrated that the left approach left less tissue than the right approach (19). Tomulescu et al. (1) demonstrated similar operative time, hospitalized length, and remission rates between the right- and left-sided VATS thymectomy. However, only patients with MG without thymoma were included in their study. Given that the position of thymoma could be on the right, left, or in the middle of the mediastinum, we speculate that selecting a better way for treatment of patients with MG, especially thymomatous ones, is important. In this study, left-sided VATS was selected for 75.0% patients with left-of-center thymoma, whereas right-sided VATS was selected for 66.7% patients with right-of-center thymoma. Surgical time, intraoperative blood loss, postoperative hospital stay, thoracic drainage volume, and postoperative complication were not significantly different among different approaches. Therapeutic effects were also not different. Thus, we recommend that VATS thymectomy could be safely and effectively performed from the either side of the thorax when the area containing all the thymus and fat tissue are dissected, as also described by Jurado et al. (14). Surgeons could select either route depending on their own experience, predominant location of thymus or thymoma, possible pleural adhesion, and concomitant operation such as pulmonary resection.
The indications for thymectomy in patients with MG remain unclear. The first and the only randomized control trial on thymectomy combined with prednisone treatment and prednisone alone is ongoing (20). However, even if thymectomy is beneficial compared with the optimized medical management, the debate persists on which kinds of patients would benefit from the operation. A recent study on the 8-year follow-up of 306 patients with MG revealed the presence of ocular MG before operation and the absence of thymoma and concomitant diseases, which are favorable factors to obtain satisfactory efficacy (21). Consistent with other studies (21,22), the present study showed that ocular MG is also found to be the only favorable predictor of clinical remission. Thymectomy remains controversial for patients with ocular MG because it is not a life threatening disease and has good response to medication. However, other scholars argued that ocular MG may severely impair the quality of life and emotion of affected patients because it presents constantly fluctuating diplopia and ptosis. Moreover, two-thirds of the cases may progress to advanced MG stages (23); as such, we are more inclined toward thymectomy for treatment of patients with ocular MG in our institute. The remission rates of patients with thymomatous MG were not significantly different from those without thymoma in the present study. We suspected that the short follow-up period of merely 2 years and mild disease component of the current study population could be the influencing factors. Other researchers also claimed that the complete remission rate of MG thymoma was not inferior to that of MG without thymoma (24). Yu et al. (25) achieved equivalent results within 40 postoperative months in their study, but the effective rates of thymomatous MG were significantly lower than nonthymomatous MG after 40 months of follow-up. They speculated that the adverse effect on thymomatous patients with MG could be due to its more severe symptoms and less responsiveness to medical treatment (25). Identification of eligible patients with MG for thymectomy should be studied in the future, as personalized therapy has received increased popularity.
One limitation of this study is that data were obtained from a retrospective database in a single institute; hence, selected and observational biases are inevitable. The limited number of patients may lead to a false-negative result. The long period spent on recruiting patients is another limitation. The learning curves of VATS thymectomy in such a long time may affect remission rates. Finally, most cases involved in this study were patients with ocular MG, which may restrict the broad application of our findings to the whole population with MG, especially for those with generalized MG. Additional prospective studies involving multiple centers are needed to confirm our results in a broader population.
In conclusion, VATS thymectomy can produce satisfactory outcomes, reduce surgical risks perioperatively, and shorten the hospitalization time compared with the conventional TS procedure. Unilateral VATS thymectomy is a clinically acceptable procedure, and can be safely and effectively performed on either side of the thorax.
Acknowledgements
The authors gratefully acknowledge the contribution of all investigators who participated in this study. They also thank the patients who participated in the study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tomulescu V, Ion V, Kosa A, et al. Thoracoscopic thymectomy mid-term results. Ann Thorac Surg 2006;82:1003-7. [PubMed]
- Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:7-15. [PubMed]
- Ashour M. Prevalence of ectopic thymic tissue in myasthenia gravis and its clinical significance. J Thorac Cardiovasc Surg 1995;109:632-5. [PubMed]
- Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653-6. [PubMed]
- Ng CS, Wan IY, Yim AP. Video-assisted thoracic surgery thymectomy: the better approach. Ann Thorac Surg 2010;89:S2135-41. [PubMed]
- Pompeo E, Tacconi F, Massa R, et al. Long-term outcome of thoracoscopic extended thymectomy for nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg 2009;36:164-9. [PubMed]
- Novellino L, Longoni M, Spinelli L, et al. "Extended" thymectomy, without sternotomy, performed by cervicotomy and thoracoscopic technique in the treatment of myasthenia gravis. Int Surg 1994;79:378-81. [PubMed]
- Yim AP. Paradigm shift in surgical approaches to thymectomy. ANZ J Surg 2002;72:40-5. [PubMed]
- Mineo TC, Pompeo E, Lerut TE, et al. Thoracoscopic thymectomy in autoimmune myasthesia: results of left-sided approach. Ann Thorac Surg 2000;69:1537-41. [PubMed]
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. [PubMed]
- Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Ann Thorac Surg 2000;70:327-34. [PubMed]
- Lo CM, Lu HI, Hsieh MJ, et al. Thymectomy for myasthenia gravis: video-assisted versus transsternal. J Formos Med Assoc 2014;113:722-6. [PubMed]
- Goldstein SD, Culbertson NT, Garrett D, et al. Thymectomy for myasthenia gravis in children: a comparison of open and thoracoscopic approaches. J Pediatr Surg 2015;50:92-7. [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [PubMed]
- Huang CS, Cheng CY, Hsu HS, et al. Video-assisted thoracoscopic surgery versus sternotomy in treating myasthenia gravis: comparison by a case-matched study. Surg Today 2011;41:338-45. [PubMed]
- Rückert JC, Walter M, Müller JM. Pulmonary function after thoracoscopic thymectomy versus median sternotomy for myasthenia gravis. Ann Thorac Surg 2000;70:1656-61. [PubMed]
- Chicaiza-Becerra LA, Garcia-Molina M, Gamboa O, et al. The cost-effectiveness of open or thoracoscopic thymectomy compared to medical treatment in managing Myasthenia gravis without thymomas. Rev Salud Publica (Bogota) 2012;14:260-70. [PubMed]
- Yim AP, Kay RL, Ho JK. Video-assisted thoracoscopic thymectomy for myasthenia gravis. Chest 1995;108:1440-3. [PubMed]
- Rückert JC, Czyzewski D, Pest S, et al. Radicality of thoracoscopic thymectomy--an anatomical study. Eur J Cardiothorac Surg 2000;18:735-6. [PubMed]
- Newsom-Davis J, Cutter G, Wolfe GI, et al. Status of the thymectomy trial for nonthymomatous myasthenia gravis patients receiving prednisone. Ann N Y Acad Sci 2008;1132:344-7. [PubMed]
- Yu S, Li F, Chen B, et al. Eight-year follow-up of patients with myasthenia gravis after thymectomy. Acta Neurol Scand 2015;131:94-101. [PubMed]
- Liu Z, Feng H, Yeung SC, et al. Extended transsternal thymectomy for the treatment of ocular myasthenia gravis. Ann Thorac Surg 2011;92:1993-9. [PubMed]
- Papatestas AE, Genkins G, Kornfeld P, et al. Effects of thymectomy in myasthenia gravis. Ann Surg 1987;206:79-88. [PubMed]
- Kim HK, Park MS, Choi YS, et al. Neurologic outcomes of thymectomy in myasthenia gravis: comparative analysis of the effect of thymoma. J Thorac Cardiovasc Surg 2007;134:601-7. [PubMed]
- Yu L, Zhang XJ, Ma S, et al. Thoracoscopic thymectomy for myasthenia gravis with and without thymoma: a single-center experience. Ann Thorac Surg 2012;93:240-4. [PubMed]


