Surgical anatomy of the tracheobronchial tree
Introduction
In the preface of his “de Humani Corporis Fabrica”, Andreas Vesalius [1514-1564] wrote that anatomy should rightly be regarded as the firm foundation of the whole art of medicine. This observation is even more pertinent to the art of airway surgery, in which safe techniques are largely dependent on optimal knowledge of normal anatomy and of its variants. Surgeons undertaking subglottic or tracheal resections must, for instance, be familiar with the particular anatomic arrangements of these structures as well as of their blood supply and innervation if they want to avoid improper operations or technical mishaps. Indeed, the essential facts of anatomy must be well known if one wants to insure that each patient gets the best possible operation for his or her airway disorder.
Anatomy of the glottis and subglottic regions
The glottis and subglottis extend from the vocal cords above to the lower border of the cricoid cartilage below. The glottis includes the vocal cords together with the anterior and posterior commissures while the subglottic region extends from a plane approximately 1 cm below the free margin of the true vocal cords to the lower border of the cricoid cartilage (1).
Surgery on the glottis and subglottis is complex not only because it is carried out in close proximity to the vocal cords but also because complete transection of the subglottic airway at any level above the cricothyroid joint will invariably result in the division of the recurrent laryngeal nerves with resultant vocal cord paralysis. In addition, the posterior rim of the upper border of the cricoid cartilage supports the arytenoid cartilages which play a critical role in vocal function (2).
Descriptive anatomy
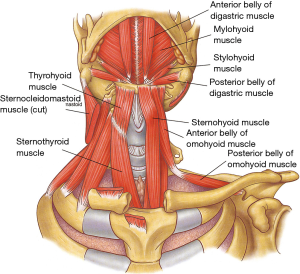
The rigid framework of the larynx is provided by nine cartilages joined by intercartilaginous membranes (Figure 1). The hyoid bone is not a true component of the larynx but is closely related to it through the extrinsic laryngeal musculature (Table 1) (Figure 2). While performing a tracheal resection, the larynx has sometimes to be released through the division of these extrinsic laryngeal muscles in order to obtain a tension-free reconstruction (3,4).

Full table
The thyroid, cricoid, and part of the arytenoid cartilages are made of hyaline cartilage whereas the other laryngeal cartilages are made of elastic fibrocartilages. With age, hyaline cartilages have a tendency to become ossified, more so and earlier in men than in women.
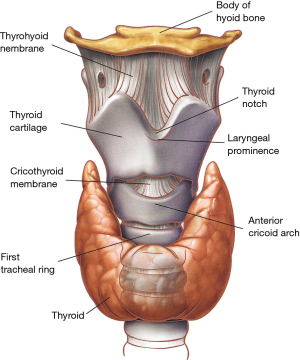
The thyroid cartilage is the largest of all laryngeal cartilages. Its superior border connects with the hyoid bone through the thyrohyoid membrane, and its inferior border is attached to the cricoid cartilage through the cricothyroid membrane (Figure 1). It is through this thick and relatively avascular membrane that the airway is closest to skin and it is where cricothyroidotomies (coniotomies) are carried out (5).
Over the anterior neck, the laryngeal prominence (Adam’s apple) is formed by the angle of union of the two lateral laminae of the thyroid cartilage at the level of C4-C5. Above the laryngeal prominence, the laminae of the thyroid cartilage diverge to create a U-shaped depression called the thyroid notch. From the posterior border of the thyroid cartilage, two slender processes extend superiorly and inferiorly, forming the superior and inferior horns (cornua), respectively.
The cricoid cartilage is the only complete cartilaginous ring of the larynx and also its main supporting structure. It has an anterior arch similar to a normal tracheal ring and a much broader posterior plate or cricoid lamina (Figure 3). The lamina has a vertical crest in its midline and two lateral fossae, sites of insertion of the posterior cricoarytenoid muscles which are the primary abductors of the vocal cords. The paired arytenoid cartilages rest on the superior border of the posterior cricoid plate (Figure 3) and articulate at the cricoarytenoid joints on the lateral part of the cricoid lamina. The vocal cords are attached posteriorly to the vocal processes of the arytenoid cartilages and anteriorly to the thyroid cartilage.

The corniculate and cuneiform cartilages are two small cylindrical or conical cartilages located above the vertex of the arytenoid cartilages in the arytenoepiglottic ligament. These cartilages pull on the epiglottis during swallowing thus contributing to the closure of the laryngeal aditus. The epiglottis is a thin, oval, and flexible lamina located in the superior part of the larynx. Anteriorly, the epiglottis is attached to the hyoid bone through the hyoepiglottic ligament, whereas its inferior end is attached to thyroepiglottic ligament. During swallowing, the superior part of the epiglottis moves backward and contributes to protect the laryngeal aditus from aspiration.
Vascular supply
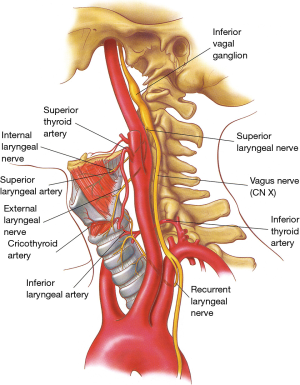
The vascular supply to the glottis and subglottic airway comes from the superior laryngeal arteries, which are branches of superior thyroid arteries, and from the posterior and inferior laryngeal arteries, which originate from the inferior thyroid artery (Figure 4). In contrast to the segmental and highly susceptible to injury blood supply of the trachea, the arterial branches going to the larynx form a rich anastomotic network and thus the larynx is somewhat resistant to ischemia.
The venous drainage of the larynx is through superior and inferior laryngeal veins, which ultimately drain into the internal jugular veins via the thyroid veins. The lymphatics of the glottis drain into the deep cervical nodes, whereas those of the subglottic airway drain into the internal jugular, prelaryngeal, and upper paratracheal nodes.
Innervation
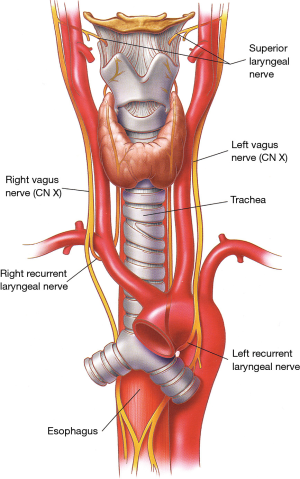
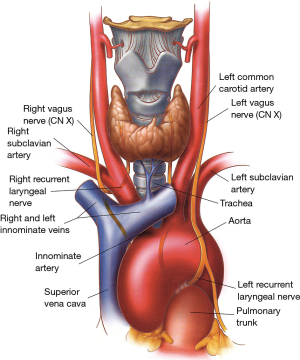
The larynx is innervated by the superior laryngeal nerves and by the inferior or recurrent laryngeal nerves, both branches of the vagus nerves (Figures 4,5).
The superior laryngeal nerves (Figure 4) divide into an internal branch, which pierces the thyrohyoid membrane and provides sensory function to the supraglottic airway, and an external branch, which provides motor function to the cricothyroid muscles and inferior constrictors of the pharynx as well as sensory function to the vocal cords (6).
At the base of the neck, the right vagus nerve crosses the origin of the right subclavian artery behind the sternoclavicular joint, and gives off the right recurrent laryngeal nerve (inferior laryngeal nerve) that loops around and under the subclavian artery (Figure 5). The right recurrent nerve ascends in the tracheoesophageal groove, where it can be injured during extensive cervicomediastinal dissections sometimes done for malignant neoplasms located near or at the thoracic inlet. On the left side, the recurrent laryngeal nerve originates close to the ligamentum arteriosum in the left chest and courses around the aortic arch from front to back before ascending to the neck in the tracheoesophageal groove.
On each side, the recurrent nerves accompany the laryngeal branch of the inferior thyroid artery behind the cricothyroid articulation (Figure 4) and enter the larynx posterior to the inferior horns of the thyroid cartilages. They give off an anterior branch, which innervates all intrinsic laryngeal muscles except the cricothyroid, and a posterior branch which supplies motor function to the inferior constrictor muscles of the pharynx.
The recurrent laryngeal nerves are susceptible to surgical injury when they enter the larynx next to the cricoid plate behind the cricothyroid articulation. This is the reason why most surgeons believe that partial or total resection of the cricoid cartilage should always be done subperichondrally as to not injure these nerves. Recurrent nerve injury at that level will produce vocal cord paralysis, the end-result of such paralysis being interference with normal phonation, respiration, and sphincteric function (paralysis of interarytenoid muscles), all associated with significant patient morbidity. The technique of subglottic resection described by Pearson and also named the “Pearson Operation” (7), allows transverse division of the airway up to the level of the inferior border of the vocal cords without injuring the recurrent laryngeal nerves.
Anatomy of the trachea
The trachea originates below the cricoid cartilage and extends from front to back to the carina. Until its anatomy and vascular supply were better understood in the late 1960’s, it was generally accepted that surgeons could safely remove no more than two or three tracheal rings, “the two centimeter rule”, and predictably be able to reconstruct the airway with primary anastomosis. As late as 1990, Professor Andreas (Andy) Naef, a prominent airway surgeon from Switzerland said: “Tracheobronchial tissue, as compared with the stomach, intestine, or even skin, does not heal well… both the rigidity and the poor blood supply of the cartilaginous structure are definitely major handicaps” (8). With improved understanding of anatomy and blood supply allowing for better use of mobilization techniques, half the tracheal length can now be safely resected and the airway be primarily reconstructed.
Descriptive anatomy
The trachea is a cartilaginous and membranous tube which is continuous with the larynx at the level of the cricoid cartilage (Figure 6). Its uppermost portion is located at the level of the sixth or seventh cervical vertebrae in the neck, while its lower end lies at the level of the fourth or fifth thoracic vertebrae in the chest. In the adult, the tracheal length ranges from 10–13 cm (longer in men than in women) with approximately 5 cm lying superior to the suprasternal notch.
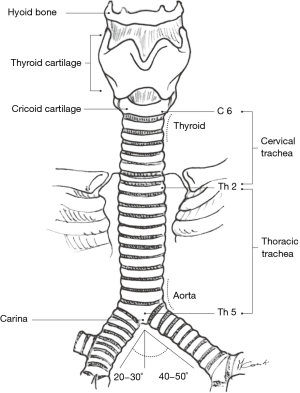
The trachea has an anterior horseshoe-shaped part made of 18−22 cartilaginous rings (2 rings per cm of trachea) and a membranous part posteriorly (Figure 7). Between the rings anteriorly, the non-cartilaginous tissue is elastic and allows lengthening or shortening of the trachea during respiration. In younger individuals, the trachea is somewhat more elastic and extensible, while in older people, it is more rigid or even sometimes ossified a significant consideration while doing tracheal resections.
The cross-sectional shape of the trachea can be elliptical (larger transverse than antero-posterior diameter), C-shaped (equal transverse and antero-posterior diameters), or U-shaped (2). In an interesting anatomical autopsy study, Mehta and Myatt showed that the U-shaped trachea was the most common variant in adult men while the elliptical shape was the most common in adult women (9). The human trachea is, however, a dynamic and distensible organ of continuously varying size, shape, and tone.
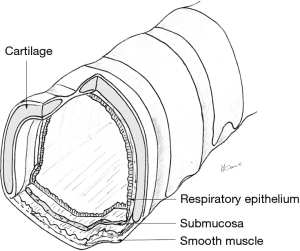
The posterior membranous tracheal wall consists of an enveloping fibrous sheath with smooth muscle. Both the tracheal cartilages and membranous wall are lined by ciliated pseudo-stratified respiratory columnar epithelium.
From the front view, the trachea is located in the midline, and from the lateral position, it courses backwards and downwards. With the neck fully extended, the trachea is half cervical and half intrathoracic but when the neck is in maximal flexion, the cricoid cartilage can be brought down to the level of the sternal notch and the trachea becomes almost entirely in an intrathoracic position. Thoracic surgeons often maintain maximal postoperative neck flexion in order to minimize anastomotic tension following segmental tracheal resection.
The anterior anatomical relationships of the trachea are those with the thyroid gland in the neck and mediastinal great vessels intra-thoracically. In the neck, the thyroid and thyroid isthmus are in front of the trachea at the level of the second or third tracheal rings while in the mediastinum, great vessels cross the trachea at various levels (Figure 8). The innominate artery crosses over the mid-trachea obliquely from its site of origin in the aortic arch and the right and left innominate veins are located anterior to the innominate artery. In young women, the innominate artery is often in a higher location at the base of the neck and can thus be in contact with a tracheal anastomosis done at neck level. This particular anatomical arrangement can sometimes lead to catastrophic postoperative tracheovascular fistulae. The superior vena cava is anterior and to the right of the trachea. Posteriorly, the membranous trachea is in contact with the esophagus on the left and vertebral bodies on the right.
Vascular supply and microcirculation
Because most complications occurring following tracheal reconstruction are related to the disruption of vascular supply at the level of the anastomosis, operating surgeons must have a clear understanding not only of the blood supply to the trachea but also of its segmental nature and longitudinal anastomotic connections.
The inferior thyroid vessels and their tracheoesophageal branches provide blood supply to the proximal trachea while the bronchial arteries vascularize the distal trachea, carina, and main bronchi (Figure 9) (10-12). The trachea is also supplied by small branches originating from the subclavian artery, internal mammary artery, and innominate artery. Once they reach the tracheoesophageal groove, the tracheoesophageal branches divide into primary tracheal and primary esophageal branches (Figure 10). Tracheal vessels enter the trachea over its lateral wall branching superiorly and inferiorly over the width of several tracheal rings.
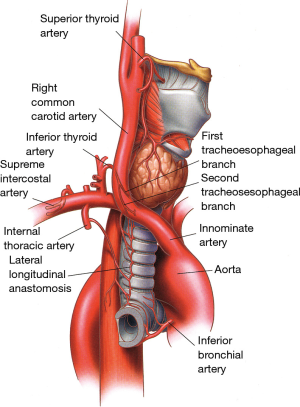
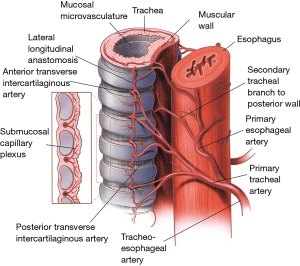
Throughout the length of the trachea, there is an extensive submucosal plexus fed by intercartilaginous arteries, each penetrating the soft tissue space between the tracheal rings and running anteriorly (Figure 10). As they reach the midline, these arteries run more deeply and terminate in submucosal capillary plexuses. The tracheal cartilages receive their blood supply from these plexuses while the membranous trachea is vascularized through secondary branches originating from the primary esophageal arteries.
Venous drainage is through the azygos and hemi azygos systems while lymphatic drainage is through the low and high paratracheal nodal chains eventually reaching the deep cervical nodes.
Innervation
The innervation of the trachea comes from tracheal branches originating from the thoracic sympathetic chain and inferior ganglion of the vagus nerve. This innervation is responsible for tracheobronchial muscle tone (bronchoconstriction or bronchodilation), mucous production, and vascular permeability. Afferent vagal fibers are also responsible for sneezing and cough reflex.
Anatomy of the carina and main bronchi
The most inferior portion of the trachea, the bifurcation, is called the carina. It lies slightly to the right of the midline at the level of the fourth or fifth thoracic vertebra posteriorly and sternomanubrial junction anteriorly.
Descriptive anatomy
The tracheal lumen narrows slightly as it progresses toward the carina. The angle between the two main stem bronchi varies among individuals and is generally greater in children than in adults (13). The configuration of the cartilages at the carina is also quite variable.
The right main stem bronchus has a vertical orientation being almost in direct line with the lower trachea (Figure 11), and its length from carina to right upper lobe take-off varies between 2.0 and 2.5 cm. By contrast, the left main stem bronchus arises at a more oblique angle and has a more horizontal orientation. The left main bronchus is approximately 4−6 cm long and it travels underneath the aortic arch to reach the posterior left hilum where it bifurcates into upper and lower lobe bronchi. Because of such an anatomical arrangement, the aorta prevents effective mobilization and elevation of the left main bronchus while doing carinal reconstructions. By contrast, the right main bronchus can easily be elevated to the level of the upper intrathoracic trachea for airway anastomosis.
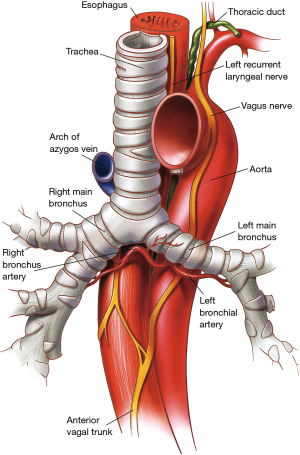
The main anatomic relationship of the carina is that with the right pulmonary artery which lies anterior and inferior to the carina making it vulnerable to injury during the course of mediastinoscopy. The left pulmonary artery arises considerably more anterior than the left main bronchus when it exits the pericardium underneath the aortic arch.
Vascular supply
Even if some of the vascular supply to the carina comes from the pulmonary arteries, most (90%) of it comes from the bronchial arteries whose level of origin, number, and distribution can be quite variable (12,14,15). More commonly, however, bronchial arteries arise from the antero-lateral aspect of the descending thoracic aorta at the level of the T5 and T6 vertebrae or from intercostal arteries located 2–3 cm distal to the origin of the left subclavian artery. There are usually three bronchial arteries, two on the left side and one on the right side (Figure 12).
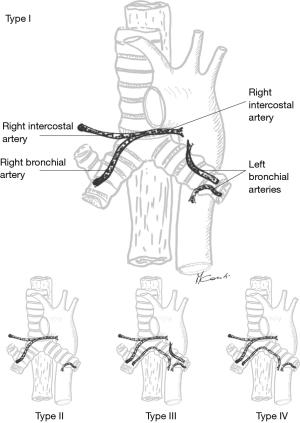
Proximally, the bronchial arteries circulate posteriorly to the airway where they lie on the membranous portion of main stem bronchi and more distally, they provide vascular supply to the lobar and segmental bronchi. On the right side, the single bronchial artery runs parallel to the azygos vein by which it is overlapped.
Most of the venous drainage from the bronchial arterial system empties into the pulmonary veins although some of it may empty into the azygos and hemiazygos system (16). Lymphatic drainage is through the subcarinal and low paratracheal nodal chains.
Main anatomical variations
There are several known anatomic variations in the tracheobronchial system but their true incidence is unknown owing to their mostly asymptomatic nature. Recognition of these variations may, however, be important while performing certain procedures such as bronchoscopy, endotracheal intubation, or positioning of lung isolation devices (17).
A tracheal bronchus is typically described as a right upper lobe bronchus originating from the trachea, usually at the junction of the middle and distal thirds. Its prevalence is in the range of 0.1% to 2% and it is often associated with cardiac congenital anomalies such as tetralogy of Fallot or ventricular septal defects. The most serious clinical implication of a tracheal bronchus is that a misplaced endotracheal tube can occlude its lumen resulting in secondary atelectasis, obstructive pneumonia or even respiratory failure (18,19). If unrecognized, accidental intubation directly into a tracheal bronchus can also lead to respiratory failure.
An accessory cardiac bronchus is a supernumerary bronchus most commonly originating from the medial wall of the bronchus intermedius and extending parallel to it toward the mediastinum. Most accessory cardiac bronchi end in a blind pouch (diverticulum), ventilated parenchyma or cystic degeneration. The only clinical implication of an accessory cardiac bronchus is that it can serve as a reservoir for infectious organisms in which case surgical resection could be indicated.
The term “bridging bronchus” is used to describe an airway malformation where the middle and right lower lobes are supplied by an aberrant bronchus originating from the left main stem bronchus and crossing over the mediastinum (17). Patients with this rare condition are often symptomatic, presenting with cough, wheezing or even respiratory distress. Most such patients, however, have associated congenital defects, usually of the cardiovascular system, which ultimately determines their prognosis.
Conclusions
Anatomically, the airway presents several unique features that account for the difficulties in the surgical management of pathological processes originating in those areas. These features include a unique cartilaginous support, its relationship to important surrounding structures, and its segmental vascular supply. It is important that the surgeon operating on the airway understands this particular anatomy, the limits of surgery, and most importantly the steps to be taken to avoid catastrophic complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thurnher D, Moukarbel RV, Novak CB, et al. The glottis and subglottis: an otolaryngologist's perspective. Thorac Surg Clin 2007;17:549-60. [PubMed]
- Maddaus M, Pearson FG. Subglottic resection. In: Pearson FG, Deslauriers J, Ginsburg RJ, et al. editors. Thoracic Surgery. New York: Churchill Livingstone, 1995;321-33.
- Montgomery WW. Suprahyoid release for tracheal anastomosis. Arch Otolaryngol 1974;99:255-60. [PubMed]
- Dedo HH, Fishman NH. Laryngeal release and sleeve resection for tracheal stenosis. Ann Otol Rhinol Laryngol 1969;78:285-96. [PubMed]
- Brantigan CO, Grow JB Sr. Cricothyroidotomy: elective use in respiratory problems requiring tracheotomy. J Thorac Cardiovasc Surg 1976;71:72-81. [PubMed]
- Noordzij JP, Ossoff RH. Anatomy and physiology of the larynx. Otolaryngol Clin North Am 2006;39:1-10. [PubMed]
- Pearson FG, Cooper JD, Nelems JM, et al. Primary tracheal anastomosis after resection of the cricoid cartilage with preservation of recurrent laryngeal nerves. J Thorac Cardiovasc Surg 1975;70:806-16. [PubMed]
- Grillo HC. Development of tracheal surgery: a historical review. Part 1: Techniques of tracheal surgery. Ann Thorac Surg 2003;75:610-9. [PubMed]
- Mehta S, Myat HM. The cross-sectional shape and circumference of the human trachea. Ann R Coll Surg Engl 1984;66:356-8. [PubMed]
- Miura T, Grillo HC. The contribution of the inferior thyroid artery to the blood supply of the human trachea. Surg Gynecol Obstet 1966;123:99-102. [PubMed]
- Salassa JR, Pearson BW, Payne WS. Gross and microscopical blood supply of the trachea. Ann Thorac Surg 1977;24:100-7. [PubMed]
- Cauldwell EW, Siekert RG, Lininger RE, et al. The bronchial arteries; an anatomic study of 150 human cadavers. Surg Gynecol Obstet 1948;86:395-412. [PubMed]
- Kubota Y, Toyoda Y, Nagata N, et al. Tracheo-bronchial angles in infants and children. Anesthesiology 1986;64:374-6. [PubMed]
- Liebow AA. Patterns of origin and distribution of the major bronchial arteries in man. Am J Anat 1965;117:19-32. [PubMed]
- Deffebach ME, Charan NB, Lakshminarayan S, et al. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis 1987;135:463-81. [PubMed]
- Marchand P, Gilroy JC, Wilson VH. An anatomical study of the bronchial vascular system and its variations in disease. Thorax 1950;5:207-21. [PubMed]
- Wooten C, Patel S, Cassidy L, et al. Variations of the tracheobronchial tree: anatomical and clinical significance. Clin Anat 2014;27:1223-33. [PubMed]
- O'Sullivan BP, Frassica JJ, Rayder SM. Tracheal bronchus: a cause of prolonged atelectasis in intubated children. Chest 1998;113:537-40. [PubMed]
- Aoun NY, Velez E, Kenney LA, et al. Tracheal bronchus. Respir Care 2004;49:1056-8. [PubMed]


