Tracheal resection and reconstruction for malignant disease
Introduction
Primary neoplasms of trachea are rare and uncommon malignancies, that account for less than 0.01% of all tumours and for about 0.2% of respiratory malignant lesion, representing a low rate of all airway neoplasm deaths (1-3).
The most common histologic types are squamous cell carcinoma (SCC), representing about 50–66% of all tracheal tumours, and adenoid cystic carcinoma (ACC), accounting for 10–15% of them (4), with wide different percentages in many clinical and epidemiological series (5-8). Several malignant histologies, of different grade, have also been described, as mucoepidermoid carcinoma, non-squamous bronchogenic carcinoma, sarcoma, carcinoid tumours, melanomas (8,9).
Squamous cell carcinoma (SCC)
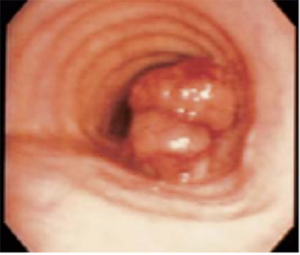
SCC usually originates from the membranous pars, with polypoid (Figure 1) or ulcerative morphology, more often in distal portion, with a peak incidence between the 6th and 7th decades. As pulmonary SCC, there is a heavy correlation with smoking habits. When local progression occurs, the tumour may infiltrate the surrounding tissue, as larynx, recurrent laryngeal nerves or esophagus, while paratracheal lymph nodes are often sites of loco-regional metastasis.
Reported 5- and 10-year survival rate is about 40–50% and 20–30%, respectively (7,10).
Adenoid cystic carcinoma (ACC)
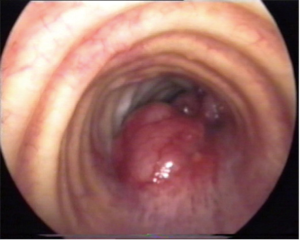
ACC may present as a polypoid lesion (Figure 2) or show an infiltrating growth of the tracheal wall, more often in proximal portion, at submucosal and perineural level, along the whole circumference or in vertical extension at any level, with a peak incidence in the fifth decade. No smoking habits correlation has been found. Distant metastases can occur more frequently than in SCC, but often show a very low growth rate.
This peculiar way of tumour spreading requires a careful attention in frozen section examination of tracheal margins.
Reported 5- and 10-year survival rate is about 65–85% and 40–55%, respectively (7,10,11).
Secondary malignant tracheal involvement may also occur by direct extension from mediastinal tumours, as haematological disorders, neoplasms of other organs like larynx, esophagus, lung, thyroid, or from pathologic cervico-mediastinal lymph nodes invasion; moreover, trachea may be the site of metastatic diffusion from distant diseases.
Clinical aspects
Dyspnoea, cough, haemoptysis and shortness of breath on effort are very common presenting symptoms, however tracheal tumours may often be underestimate for months or years, especially in case of ACC, mucoepidermoid carcinoma or carcinoid, due to their silent and slow-growing features (12).
They are commonly misdiagnosed as asthma, but when the lumen progressively narrows, patient will develop dyspnoea at rest, hoarseness, wheezing, stridor, revealing an airway obstruction, that may rapidly become a life-threatening condition (13,14).
Other symptoms may be dysphonia from recurrent laryngeal nerve involvement, recurrent pulmonary infections from distal obstruction or dysphagia from esophageal invasion (15).
Diagnostic imaging
Standard chest radiography is often the first imaging study requested in patients with such respiratory symptoms, but signs of tracheal tumours, as obstruction of air column, can be identified in only 18–28% of affected patients (16).
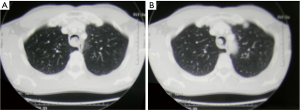
Neck and chest computed tomography (CT) (Figure 3) is still the most accurate and useful method to evaluate the intra- and extra-luminal extension of disease, the depth of invasion, the involvement of adjacent organ and pathological lymph nodes, and the presence of distant or local metastasis (17).
Positron emission tomography (PET), as in lung cancer staging, may be helpful to detect local and distant metastasis and to better evaluate the real extension of neoplasm, particularly in SCC, related to its high metabolic activity (17).
Endoscopic evaluation
Tracheo-bronchial evaluation under general anesthesia with rigid and flexible endoscopy is the most useful test in diagnosing and evaluating tracheal neoplasms.
Endoscopy, that should be performed or attended by the first surgeon, allows to carefully evaluate the intraluminal extent of disease, the vocal cords condition, the length of normal trachea and airway diameter, the relationship with cricoid cartilage and carina and to confirm the histologic diagnosis (15,18).
Surgical treatment
Surgical tracheal resection, radiation therapy and endoscopic resection represent the most important treatment options in tracheal tumours, however complete surgical resection is still the best procedure to achieve a long-term survival in selected cases, where no contraindications have come to evidence (14).
Main contraindications to surgery are represented by an excessive length in tumour extension, that would not allow to perform a safe, tension-free anastomosis, presence of distant metastasis and the involvement of surrounding, not resectable, organs or tissues, severe comorbidities as, for example, active steroid use, not balanced diabetes mellitus or serious nutritional impairment (19).
Surgical approaches and incisions
The choice of way of access represents a fundamental step in surgical planning, where a wrong decision can make the procedure extremely difficult or impossible (20).
Several point should be focused by the surgical team to take the right decision:
- Site and exact extension of the tumour, into and outside of the airway;
- Amount of normal trachea that will remain after the planned resection;
- Age and the body complexion, where an old, kyphotic patient with a short, stiff neck, in whom a resection of no more than 4 cm will be accepted, is a completely different candidate from a young, thin, tall patient, who may undergo up to 6 cm resection (21,22);
- History of previous cervico-thoracic surgery, infection or irradiation.
Upper tracheal tumour
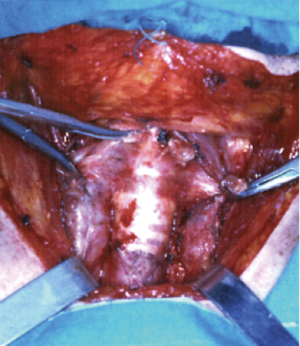
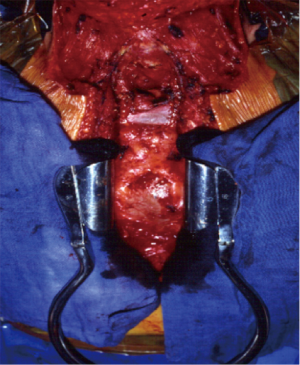
Tumours in the upper portion of trachea will be dealt by a low collar cervical incision (Figure 4) with a draped field also for a possible extension along the sternum and right hemithorax.
Middle tracheal tumour
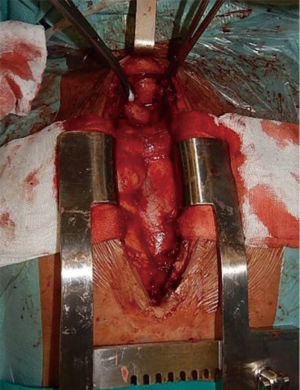
Tumours in the middle portion of trachea will be approached by a combined cervical incision, followed by an upper sternal split (Figure 5), with a possible further extension in a full sternotomy (Figure 6) or right thoracotomy. This approach will also allow to perform the right, or bilateral, hilar intrapericardial release, while the laryngeal release is rarely needed.
Lower tracheal tumour
Tumours in the lower portion of trachea will be approached through the chest, by a postero-lateral right thoracotomy in 4th intercostal space.
Anterior approach
Low collar incision, with or without partial sternal section, is performed in the standard fashion with patient usually placed in supine position with a folded pillow or an inflatable bag under the shoulders to allow a controlled neck extension and flexion, that will be needed in the different steps of surgical procedure.
Skin incision, preparation and lifting of muscle-cutaneous flap, initial careful tracheal exposure, protection of recurrent laryngeal nerves, cross-field ventilation and placement of lateral, traction sutures are performed following the standard criteria used in management of tracheal stenosis (23).
Tracheal dissection is carefully performed with the same attention, but, in neoplastic disease, starting far from the tumour borders, in order to avoid tumour dissemination, to identify the correct surgical planes and to evaluate possible involvement of surrounding organs, as larynx, prethyroid muscles, esophagus or thyroid.
Another important issue is the avoidance of an excessive extension of loco-regional nodal dissection, due to the high risk of disruption of vascular supply of the tracheal wall.
Once the resection is planned, frozen section examination of proximal and distal margins is fundamental, but often, especially in ACC, where the submucosal, longitudinal extension of neoplasm can be found well distant from visible disease, a compromise may be necessary and a microscopical tumour involvement of section margins may be accepted to avoid an excessive tension of the anastomosis.
Also during reconstruction, we perform anastomosis following the Grillo criteria for the anterior wall, using interrupted polyglycolic (Vicryl) 3-0 suture, but with a different technique for membranous wall, where we prefer a running suture with long lasting absorbable 4-0 polydioxanone (PDS) (24).
At the end of the procedure, a thick suture between the chin and the chest (the so-called guardian suture) is passed, to block accidental postoperative neck hyperextension, and left in place for about seven days.
Transthoracic approach
After the right postero-lateral thoracotomy in fourth intercostal space, a complete dissection of middle and lower trachea, esophagus, carina and right pulmonary hilum will be feasible (25).
Intraoperative ventilation, after tracheal section and resection, is conducted by a cross-field armored tube or, as option, with high-frequency jet ventilation, while reconstruction will follow the same steps of anterior approach.
This approach allows to proceed with section of inferior pulmonary ligament and right intrapericardial hilar release, that would increase the mobility of the lower trachea-bronchial structures, reducing the tension on the suture.
As our predilection in every intrathoracic bronchoplastic or anastomotic procedure, like bronchial or tracheo-bronchial sleeve resection or in lung transplantation, a viable tissue flap, in most of the cases a thymic-pericardial fat pad, will be prepared, passed behind the superior vena cava and fixed around the anastomosis, to provide healing factors and to separate the suture from the surroundings vessels.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nouraei SM, Middleton SE, Nouraei SA, et al. Management and prognosis of primary tracheal cancer: a national analysis. Laryngoscope 2014;124:145-50. [PubMed]
- Junker K. Pathology of tracheal tumors. Thorac Surg Clin 2014;24:7-11. [PubMed]
- Thompson AD, Talavari Y, Mehari A, et al. Tracheal cancer mortality and trends in the United States. Internet J Oncol 2014;10:1-2.
- Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol 2011;34:32-7. [PubMed]
- Honings J, van Dijck JA, Verhagen AF, et al. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol 2007;14:968-76. [PubMed]
- Yang KY, Chen YM, Huang MH, et al. Revisit of primary malignant neoplasms of the trachea: clinical characteristics and survival analysis. Jpn J Clin Oncol 1997;27:305-9. [PubMed]
- Regnard JF, Fourquier P, Levasseur P. Results and prognostic factors in resections of primary tracheal tumors: a multicenter retrospective study. The French Society of Cardiovascular Surgery. J Thorac Cardiovasc Surg 1996;111:808-13; discussion 813-4. [PubMed]
- Webb BD, Walsh GL, Roberts DB, et al. Primary tracheal malignant neoplasms: the University of Texas MD Anderson Cancer Center experience. J Am Coll Surg 2006;202:237-46. [PubMed]
- Shadmehr MB, Farzanegan R, Graili P, et al. Primary major airway tumors; management and results. Eur J Cardiothorac Surg 2011;39:749-54. [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 2004;78:1889-96; discussion 1896-7.
- Maziak DE, Todd TR, Keshavjee SH, et al. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg 1996;112:1522-31; discussion 1531-2. [PubMed]
- Macchiarini P. Primary tracheal tumours. Lancet Oncol 2006;7:83-91. [PubMed]
- Honings J, Gaissert HA, van der Heijden HF, et al. Clinical aspects and treatment of primary tracheal malignancies. Acta Otolaryngol 2010;130:763-72. [PubMed]
- Sherani K, Vakil A, Dodhia C, et al. Malignant tracheal tumors: a review of current diagnostic and management strategies. Curr Opin Pulm Med 2015;21:322-6. [PubMed]
- Grillo HC. Primary tracheal neoplasms. In: Grillo HC. Surgery of the trachea and bronchi. Hamilton, London: BC Decker Inc, 2004:207-48.
- Honings J, Gaissert HA, Verhagen AF, et al. Undertreatment of tracheal carcinoma: multidisciplinary audit of epidemiologic data. Ann Surg Oncol 2009;16:246-53. [PubMed]
- Wu CC, Shepard JA. Tracheal and airway neoplasms. Semin Roentgenol 2013;48:354-64. [PubMed]
- Honings J, Gaissert HA. Tumours of the trachea. In: Kuzdzat J, editor. ESTS Textbook of thoracic surgery. Cracow: Medycyna Praktyczna, 2014:359-71.
- Wright CD. Tracheal resection. In: Kuzdzat J, editor. ESTS Textbook of thoracic surgery. Cracow: Medycyna Praktyczna, 2014:420-7.
- Grillo HC. Surgical approaches. In: Grillo HC. Surgery of the trachea and bronchi. Hamilton, London: BC Decker Inc, 2004:507-16.
- Gaissert HA, Honings J, Gokhale M. Treatment of tracheal tumors. Semin Thorac Cardiovasc Surg 2009;21:290-5. [PubMed]
- Li Y, Peng A, Yang X, et al. Clinical manifestation and management of primary malignant tumors of the cervical trachea. Eur Arch Otorhinolaryngol 2014;271:225-35. [PubMed]
- Grillo HC. Tracheal reconstruction: anterior approach and extended resection. In: Grillo HC. Surgery of the trachea and bronchi. Hamilton, London: BC Decker Inc, 2004:517-48.
- Rea F, Callegaro D, Loy M, et al. Benign tracheal and laryngotracheal stenosis: surgical treatment and results. Eur J Cardiothorac Surg 2002;22:352-6. [PubMed]
- Grillo HC. Reconstruction of the lower trachea (transthoracic) and procedures for extended resection. In: Grillo HC. Surgery of the trachea and bronchi. Hamilton, London: BC Decker Inc, 2004:587-98.