Management of large mediastinal masses: surgical and anesthesiological considerations
Introduction
The mediastinum is a site for a wide range of various neoplasms, with often rare histologies (1,2), imposing both diagnostic and therapeutic challenges. Particularly large mediastinal masses, or intrathoracic masses with mediastinal compression, offer surgical challenges, due to the complex anatomy of the mediastinum, with obstruction, compression, or invasion of vital surrounding structures. In addition, anesthesiological management during surgery can be complicated by the so-called “mediastinal mass syndrome” (MMS) (3), characterized by acute respiratory and hemodynamic decompensation, due to mechanical compression of mediastinal structures. Therefore, meticulous preoperative assessment, preparation and collaboration between the surgeon and anesthesiologist are essential.
In this paper, we discuss considerations and recommendations regarding perioperative preparation and management in patients with large masses involving or compressing the mediastinum (Figure 1). Due to the rarity of mediastinal tumors, especially large masses, little literature is available regarding this matter. The current manuscript is based on a review of the literature and our own clinical experience.
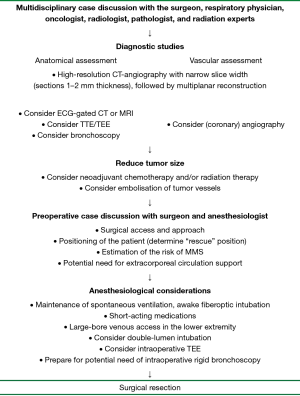
Preoperative preparation
Tumors involving the mediastinum include a wide variety of histologies, both benign and malignant in nature. Differential diagnoses can be divided based upon the localization in the three different mediastinal compartments. Excellent reviews are available regarding the various approaches for distinguishing and diagnosing different mediastinal tumors (1,2), and this subject is beyond the scope of this paper.
Roughly 40% of all tumors involving the mediastinum are asymptomatic at presentation (4). Consequently, these masses regularly grow to such a size that determination of the site of origin is challenging. Regardless of the definitive diagnosis, the exact size or location, all large mediastinal and intrathoracic masses may cause mechanical compression of vital mediastinal structures, purely due to the sheer weight and volume of the tumor. Acute or chronic respiratory insufficiency may occur with compression of the airways, i.e., trachea, bronchi or both (5). Hemodynamic decompensation can be caused by compression of the heart or major vessels in the mediastinum. These effects, also called MMS, can be greatly aggravated by induction of anesthesia (3) or positional changes during surgery. The exact incidence of perioperative MMS remains unknown, and reporting is limited to isolated cases (3). Nevertheless, insufficient preoperative preparations and inadequate perioperative management can promptly lead to life-threatening situations (3). Therefore, careful diagnostic workup and detailed preoperative imaging is essential in all cases to assess the anatomy and exact relationship of the tumor with the surrounding vital mediastinal structures. Furthermore, as the size of the tumor can both complicate surgical resection and anesthetic management, effort should be undertaken to reduce tumor volume before definitive operative therapy.
Anatomical assessment of the mediastinum
Traditionally, following chest radiography, computed tomography (CT) of the chest is the initial choice of imaging modality in the evaluation of mediastinal masses (6). It is valuable in demonstrating the morphology of the tumor, its exact anatomical location, and its relation with structures in the mediastinum. We prefer the use of high-resolution CT-angiography with narrow slice width (sections 1–2 mm thickness), followed by multiplanar reconstruction. The use of intravenous contrast is important in delineating the vasculature of the tumor and its direct surroundings.
While CT is superior in spatial resolution, detection of calcification and bony destruction, magnetic resonance imaging (MRI) is more adequate in soft tissue differentiation and delineating tissue boundaries. MRI has been suggested to be more accurate in diagnosing invasion of the mediastinum (7,8) and chest wall (8) when compared to CT. Furthermore, blood vessels are identifiable without the need for intravenous contrast enhancement, and could be helpful in patients who cannot receive iodinated intravenous contrast.
The heart takes a central position in the mediastinum. Subsequently, cardiac motion artifacts may cause misinterpretation of the images produced by standard CT or MRI, especially in evaluating structures close to the heart. Recently, the advance of electrocardiogram (ECG)-gated cardiac CT and ECG-gated cardiac MRI has produced superior image quality with nearly artifact-free images, greatly improving the assessment of cardiac tumors and its relations to adjacent structures. In addition to these static images, dynamic MRI with dynamic cinematic displays of cardiac motion can provide essential complementary information regarding cardiac involvement or tumor invasion (9). Both cardiac CT and cardiac MRI have been recommended by the American College of Cardiology Foundation for clinical evaluation of cardiac masses, extracardiac structures, and involvement and characterization of these masses (10,11). Conversely, the relationship of central mediastinal tumors with the heart and cardiac structures can potentially be much more reliably evaluated with these new imaging techniques.
Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) are standard diagnostic imaging modalities in the perioperative setting of cardiac surgery. For the evaluation of mediastinal masses, its role is less defined. However, with the central position of the heart in the mediastinum, large mediastinal tumors can cause significant compression or even invasion of the heart (12-14). Echocardiography offers complimentary information to CT and MRI images when assessing encroachment or compression of cardiac structures by the mediastinal mass (15), and when distinguishing between pericardial and intracardiac tumors. In any case, we advise to perform a routine preoperative TTE to evaluate cardiac structure and function as part of preoperative risk assessment.
Fluorodeoxyglucose positron emission tomography (FDG-PET) has an increasing role in the diagnostic pathway evaluating mediastinal tumors (16), with impact on diagnosis, staging and prognosis. However, FDG-PET adds little additional information regarding the anatomical assessment of the mediastinum.
Regarding invasive diagnostic procedures, bronchoscopy is an important tool, both in the preoperative assessment as in the operating room. Flexible bronchoscopy can visualize the trachea, proximal airways, and segmental airways to evaluate potential extrinsic airway compression from large mediastinal masses. If airway compression is present, the patient usually has a position preference. Anticipating the positional changes during surgery, the severity of airway compression in different positions can be evaluated during bronchoscopy.
Vascular assessment of the mediastinum
Large tumors, especially in the mediastinum, can be highly vascularised, complicating surgical resection. For example, in a series of 34 patients with a solitary fibrous tumor (SFT) (17), 35% had large collateral feeding vessels which could be seen on CT, MRI, and angiography. Blood supply to mediastinal masses has been reported to be highly variable, possibly derived from branches from the internal mammary artery (18-20), bronchial arteries (19), thyrocervical trunk (19,20), intercostal vessels (21), phrenic nerve vessels, pulmonary artery, and even from coronary arteries (22-26). These large feeding vessels may cause technical and bleeding complications during surgical resection. Therefore, it is advisable to be maximally informed regarding the vascularisation prior to surgery, either through MRI or high-resolution CT with intravenous contrast with multiplanar reconstruction. Angiography, i.e., pulmonary angiography or occasionally coronary angiography is also a valuable diagnostic modality to assess the vasculature of large tumors involving the mediastinum. In addition, during angiography, embolization of these feeding vessels can be performed before operative treatment, and can theoretically be useful in order to reduce blood loss during surgery. This has been done in several reported cases (17,19,20,22,27,28), although no definitive evidence exists that this will indeed lower perioperative bleeding complications.
In the setting of compression or invasion of the vena cava by large mediastinal tumors, the venous circulation will be maintained through collateral vessels (29,30). Similarly to large feeding vessels, the presence of large venous collaterals can also cause significant surgical bleeding complications. Again, contrast-enhanced, high-resolution CT with multiplanar and 3D reconstruction is an excellent technique in portraying the collateral circulation (31) to facilitate an optimal surgical strategy.
Reducing tumor size
Depending on the histological diagnosis, neoadjuvant therapy can be important in reducing tumor mass and surrounding infiltration, potentially leading to resectability in initially inoperable cases, or greatly facilitating surgical resection. For instance, for primary mediastinal nonseminomatous germ cell tumors, multimodality treatment is the approach of choice, consisting of neoadjuvant chemotherapy followed by surgical resection of residual disease (32). In thymomas, neoadjuvant chemotherapy and radiotherapy is successful in achieving volume reduction preoperatively (33). A recent survey among members of the European Association for Cardio-Thoracic Surgery revealed that neoadjuvant therapy was used by most surgeons in the treatment of thymoma, and is significantly more utilized by high-volume surgeons (34).
Making a histopathological diagnosis through fine-needle aspiration (FNA) or core needle biopsy is important in guiding decision-making in this regard. Various methods are available (35), depending on the location of the mass, including TTE approaches with image-guidance by CT (36) or ultrasound, guidance by endobronchial (37) and transesophageal ultrasound, or surgical methods including mediastinoscopy and mediastinostomy. However, the role of obtaining tissue diagnosis in the evaluation of mediastinal masses remains to be controversial due to the fear of tumor cell seeding in the biopsy tract, especially when thymic neoplasms are likely (38,39). As proposed by the National Comprehensive Cancer Network Guidelines on thymic malignancies (39), surgical biopsy should be avoided if a resectable thymoma is strongly suspected based on clinical and radiologic features. If biopsy is performed, transpleural approaches should be avoided.
Furthermore, as mediastinal tumors often comprise rare histological entities, evidence is not always available to guide the utilization of neoadjuvant therapy, even when a pathological diagnosis is available. In this regard, FDG-PET has become an increasingly important tool. Although the additional anatomical information obtained by FDG-PET is insufficient, the metabolic activity of the mass has been shown to predict the response of the tumor to neoadjuvant therapy in various malignancies (40-43). Furthermore, cellular markers for the level of proliferation activity (like mitotic and apoptotic index) could also be used to predict response to neoadjuvant therapy (44). Decisions about the value of neoadjuvant therapy should be based on multidisciplinary discussion with the surgeon, respiratory physician, oncologist, radiologist, pathologist, and radiation experts.
As mentioned earlier, embolisation of afferent vessels has been performed in order to reduce surgical bleeding complications. However, whether this technique can also reduce tumor size and volume remains to be investigated. Uterine artery embolization for symptomatic uterine fibroids has been reported to be effective (45), resulting in infarction of fibroids, with reduction of volume and complaints. Furthermore, chemoembolisation has become an accepted treatment option for hepatocellular carcinoma, usually as a palliative technique (46). It involves the combined intra-arterial administration of cytotoxic anticancer drugs and embolisation agent into a liver tumor, inducing extensive tumor necrosis. Whether these embolisation techniques could be successful as a neoadjuvant tumor reduction modality in large mediastinal tumors, remains to be an interesting area for future research.
Anesthesiological considerations
Patients with large mediastinal masses pose significant anesthesiological challenges due to the possible occurrence of MMS (3,47). Prior to definitive surgical resection, a thorough perioperative plan should be made between the surgeon and anaesthesiologist (3,47,48). Preoperative risk assessment by the anesthesiologist is essential, based on clinical, functional, bronchoscopic, and radiological data, with emphasis on the anatomical details of the tumor and its relation with the surroundings. Detailed history taking could reveal symptoms of airway compromise such as cyanosis, stridor, dyspnea at rest, postural dyspnea and orthopnea. Cardiovascular symptoms may result from compression of the great vessels or the heart itself. All these symptoms should be evaluated prior to surgery in different positions with the patient awake, especially the supine and lateral decubitus position. It is important to determine a “rescue position”—the position the patient finds the most comfortable in terms of complaints, with the most ideal respiratory and hemodynamic functioning.
After preoperative assessment, patients may be distributed into different risk categories (3). Low risk patients are asymptomatic or have minimal symptoms. Intermediate risk patients have mild to moderate postural symptoms, and tracheobronchial compression <50%. High risk patients include patients with severe postural symptoms, tracheobronchial compression >50% or tracheobronchial compression with associated hemodynamic symptoms. This risk stratification may guide the utilization of perioperative pre-emptive measures and precautions, like the safest form of anesthesia, the optimal positions for induction of anesthesia and surgery, the additional value of intraoperative (rigid) bronchoscopy and TEE, and the potential assistance of extracorporeal circulation (ECC). Furthermore, due to the possible involvement of the superior vena cava (SVC) in large mediastinal masses, large-bore venous access should be secured in the lower extremity (preferably the femoral vein) rather than the upper extremity in all patients, regardless of risk category. Also, from the surgeon’s point of view, we prefer double-lumen endobronchial intubation whenever feasible, as intentional unilateral pulmonary collapse could greatly facilitate tumor dissection in most cases.
In general, low risk patients tolerate general anaesthesia without problems. Patients with intermediate or high risk need an individualized approach. Our preferred approach for airway management is maintenance of spontaneous ventilation, combined with awake fiberoptic intubation. Furthermore, with the fiberoptic scope, airway collapse or obstruction can be assessed. This can be performed in various positions of the operating table in order to further determine the optimal rescue position. Finally, the use of short-acting medications is recommended for prompt and precise control of the level of general anesthesia (3,47).
When intraoperative acute respiratory and hemodynamic decompensation does occur, and the possibility of a surgical bleeding issue has been properly addressed, mechanical compression of vital mediastinal structures should be relieved immediately. First of all, the operating table should be rapidly tilted to the before determined rescue position. In the event of airway compression or obstruction, rigid bronchoscopy has been suggested as a method to secure the airway past the site of the problem. For initial hemodynamic stabilisation, the basic principles of cardiac tamponade treatment should be followed, including the administration of appropriate fluids, vasopressive and inotrope medication. Furthermore, always consider the surgeon’s ability to elevate the mass physically from the compromised structures.
The definite rescue modality when MMS occurs is ECC, providing an avenue for both oxygenation and circulatory support. Earlier reports have described “stand-by” ECC as a rescue measure (49-51) for intraoperative hemodynamic or respiratory deteoriation. However, even with a primed ECC circuit and perfusionist present in the operating room, it will still take 5–20 minutes before emergency cannulation of the femoral vessels is performed and ECC is initiated (48); an interval likely to result in significant neurological injury. Therefore, several authors have recommended femoral cannulation under local anesthetics and initation of ECC before induction of anesthesia (3,48), especially in patients with high risk of MMS and are deemed “uncertain” or “unsafe” according to the classification proposed by Erdös and Tzanova (3,48).
Surgical approach
Approach
Various surgical approaches are available to access the mediastinal and thoracic cavity. In the surgical management of large mediastinal tumors, a midline approach through a sternotomy, or a lateral approach through a thoracotomy are most often utilized, and less frequently a classic clamshell or hemi-clamshell incision. Although resection through robot-assisted and video-assisted thoracic surgery (VATS) have become increasingly popular (52,53), these techniques seem unsuitable for large masses (53), especially for tumors larger than 10 cm (53).
In our center, we advocate a midline approach through a median sternotomy whenever feasible. Median sternotomy, with the patient in supine position, is the gold standard for most cardiac operations, and has become increasingly popular for bilateral pulmonary procedures, with proper exposure of the hilar structures. It provides a wider surgical field for the approach of the anterior mediastinum when compared to an anterior or anterolateral thoracotomy. This midline approach can be extended to a hemiclamshell incision, combining a partial vertical median sternotomy with an anterior thoracotomy, for even better visibility of especially the posterior hilum, including the lower lobe. In the majority of cases, we believe this approach is favorable comparing to a lateral approach regarding positioning of the patient, proximal central vascular control, and the ease of connecting ECC. Although cardiac compression may occur in the supine position in the setting of large tumors of the anterior mediastinum, this should have become apparent with history taking and during routine physical examination in the ward, and appropriate precaution measurements can be undertaken. In these cases, it is feasible to cannulate the femoral vessels under local anesthesia in readiness for ECC, even before induction of anesthesia. Furthermore, anticipating the need for ECC for hemodynamic support in the occurrence of MMS, the supine position would also greatly facilitate the approach to the femoral vessels for cannulation.
In addition to the positional advantages and ease of connection to ECC in the supine position, a midline approach also facilitates the access to the hilar structures for both proximal vascular control and dissection of the anterior extent of mediastinal tumor. During dissection of large mediastinal masses, feeding vessels could tear due to sheer stress caused by manipulation, leading to serious hemorrhagic complications which are hard to control. Appropriate precautions can be undertaken, based on the information provided by preoperative multimodality imaging regarding the vascular anatomy of the tumor. In these cases, intrapericardial or proximal control of the main pulmonary artery and pulmonary veins is easier through a midline approach, and may be necessary before more peripheral dissection can be started.
When the tumor extends to the pleural cavity, exposure can be improved by applying a retractor to the sternal edge (54), which is commonly used during internal mammary artery harvesting for coronary artery bypass grafting (CABG). This allows constant upward and outward retraction of the chest wall. For optimal stabilisation of large mediastinal masses, heart positioners can be used which are popularized for use in off-pump CABG procedures for stabilisation of the heart through suction devices. When used in the setting of large mediastinal masses (55), damage to the tumor by manipulation of surgical instruments can be minimalized, while providing a stable retraction of the mass, and reducing the risk of sheer stress on feeding vessels.
Extracorporeal circulation (ECC)
From the surgeon’s point of view, the assistance of ECC can greatly facilitate dissection of the tumor, with the possibility to safely deflate the lungs and to retract the heart, enhancing exposure of the tumor. Also, greater manipulation of the tumor can be achieved without the risk of hemodynamic or respiratory consequences due to compression of vital mediastinal structures. However, the need for systemic heparinization during ECC could increase the risk of hemorrhagic complications during dissection of the tumor. Therefore, a case-by-case preoperative risk assessment should be made to assess the need of ECC, and in which period of the surgical setting.
Cannulation of the ascending aorta, right atrium or both superior and inferior vena cava, as routinely used in most cardiac procedures, seem inappropriate during surgery for large mediastinal masses. Involvement of cardiovascular structures of the mediastinum is common in this setting, complicating exposure of the usual sites for central cannulation. Furthermore, the cannulas needed for ECC could become cumbersome and obstructive for the surgeon during prolonged tumor dissection. Therefore, peripheral cannulation of the femoral vessels (3,48,50) is advised when ECC is deemed necessary or helpful.
From the oncologist’s point of view, the use of ECC during oncological surgery is poorly reported. A recent systematic review of the use of ECC during surgical resection for patients with locally advanced lung cancer yielded only 72 patients (56). They found that extended resections of locally advanced lung cancer is feasible with the help of ECC, although beforehand planning of the use of ECC was associated with significantly higher survival than cases where unplanned or emergent placement of ECC was necessary.
Involvement of the SVC
All tumors involving the mediastinum, especially large masses, have the potential to involve or invade the great vessels within this confined space. Of these great vessels, the SVC is the most commonly affected (57,58), with varying extent of involvement, ranging from partial compression or invasion, to the development of a SVC syndrome (59). The exact incidence of SVC involvement in mediastinal tumors is unknown, due to the relative rareness of this pathology. Several small case series have reported an occurrence rate of 10% in mediastinal seminomas (60). In a series of 89 patients with invasive primary mediastinal tumors who underwent surgical resection, 24% underwent resection of the SVC (57). In a more recent multi-institutional Italian series with 249 patients with stage III thymic tumours, 12.4% had preoperative SVC involvement. Traditionally, involvement of great vessels by thoracic tumors has long been considered inoperable (61), with infavorable long-term outcomes. However, with improving techniques, resection and reconstruction of the SVC is nowadays the most accepted extended vascular resection for mediastinal great vessel involvement. However, detailed preoperative and operative planning is required.
Depending on the extent of involvement, partial resection of the SVC can be performed or even complete resection followed by reconstruction with prosthetic grafts or vein grafts. Spaggiari advocated partial resection if less than 50% of the circumference of the SVC is affected, with pericardial patch repair when needed (62). Although partial resection can be performed with partial clamping alone when SVC involvement is minimal, crossclamping of the SVC is the most utilized technique for reconstruction (63). Systematic heparinization should be initiated before clamping (57,62,63). However, SVC crossclamping is associated with important hemodynamic compromise in 30% of patients (63). In this setting, intraoperative vasoconstrictive agents and appropriate fluid loading are important in increasing venous return to the heart and maintaining intracranial cerebral perfusion pressure (57,62,63). Correspondingly, angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists should be discontinued preoperatively to avoid intraoperative hypotension and hypovolemia. Moreover, SVC crossclamping can cause cerebral ischemia even without observable changes in blood pressure or venous oxygen saturation (64). Therefore, when SVC involvement is suspected preoperatively, we recommend standard additional monitoring modalities to assess adequacy of cerebral perfusion, preferably cerebral oximetry with near-infrared spectroscopy (NIRS) (64,65) or transcranial doppler (66), in combination with simultaneous monitoring of jugular vein pressure (65).
The time limit to safely perform SVC crossclamping remains unclear. Various authors have reported that crossclamping of the SVC can be performed for 60 minutes (61,67) and even up to 100 minutes (67) without neurological complications, with or without pre-existing SVC obstruction. However, we prefer the application of a simple, passive bypass technique from the innominate vein to the right atrium with a heparin-coated shunt (Gott shunt) (68), obviating the need for additional heparinization. Because clamping of the SVC raises the pressure gradient between the innominate vein and the right atrium, a pumping device is not necessary. This simple technique avoids hemodynamic instability and cerebral ischemia due to venous hypertension.
Organization of care
Centralisation of care for patients with rare malignancies has been advocated (69) in order to improve quality of care and outcome. This should also apply for mediastinal tumors, especially regarding large masses in the mediastinum. As discussed before, the preoperative workup and intraoperative management of these tumors is challenging, and a dedicated multidisciplinary approach is essential. Concentration in specialized centers is important for the development of expertise, and the facilitation of training and research. These intended centers should have regular multidisciplinary team meetings with the presence of surgeons, oncologists, radiologists, pathologists, and radiation experts specialized in respiratory medicine. A team of anesthesiologists should be available, dedicated to perioperative cardiovascular and cardiothoracic care. With the potential need for ECC, the operation should be headed by cardiothoracic surgeons or thoracic surgeons with cardiac surgery on-site.
For example, in the treatment of thymoma, it has been noted that high-volume surgeons are significantly more often working with dedicated anesthesiologists, and are more likely to have access to a tissue bank for research purposes (34). However, an exact cutoff value for high-volume surgeons or centers remains to be elucidated.
Conclusions
In the perioperative management of surgical treatment of large mediastinal masses or intrathoracic tumors with mediastinal compression, attention to anatomical details of the tumor and it relations with vital mediastinal surrounding structures is essential. Preoperative preparation measures include appropriate preoperative multimodality imaging, with emphasis on the vascular anatomy of the tumor. Multidisciplinary team discussions should assess whether neoadjuvant therapy can be beneficial on a case-by-case basis. Furthermore, an assessment needs to be made by the anesthesiologist to evaluate the risk of MMS. With adequate preoperative team planning, a safe anesthesiological and surgical strategy can be realized. The management of these tumors is challenging and should be concentrated in specialized centers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. [Crossref] [PubMed]
- Kim JY, Hofstetter WL. Tumors of the mediastinum and chest wall. Surg Clin North Am 2010;90:1019-40. [Crossref] [PubMed]
- Erdös G, Tzanova I. Perioperative anaesthetic management of mediastinal mass in adults. Eur J Anaesthesiol 2009;26:627-32. [Crossref] [PubMed]
- Davis RD Jr, Oldham HN Jr, Sabiston DC Jr. Primary cysts and neoplasms of the mediastinum: recent changes in clinical presentation, methods of diagnosis, management, and results. Ann Thorac Surg 1987;44:229-37. [Crossref] [PubMed]
- Hsu AL. Critical airway obstruction by mediastinal masses in the intensive care unit. Anaesth Intensive Care 2013;41:543-8. [PubMed]
- Takahashi K, Al-Janabi NJ. Computed tomography and magnetic resonance imaging of mediastinal tumors. J Magn Reson Imaging 2010;32:1325-39. [Crossref] [PubMed]
- Webb WR, Gatsonis C, Zerhouni EA, et al. CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology 1991;178:705-13. [Crossref] [PubMed]
- Biederer J, Mirsadraee S, Beer M, et al. MRI of the lung (3/3)-current applications and future perspectives. Insights Imaging 2012;3:373-86. [Crossref] [PubMed]
- Pozzoli A, Klinkenberg TJ, De Maat GE, et al. Cardiac dynamic magnetic resonance of a giant lung carcinoma invading the left atrium: do not let the imaging fool you. Eur J Cardiothorac Surg 2013;44:377-8. [Crossref] [PubMed]
- Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. Circulation 2010;122:e525-55. [Crossref] [PubMed]
- American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG, Bluemke DA, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation 2010;121:2462-508. [Crossref] [PubMed]
- Asteriou C, Barbetakis N, Kleontas A, et al. Giant mediastinal teratoma presenting with paroxysmal atrial fibrillation. Interact Cardiovasc Thorac Surg 2011;12:308-10. [Crossref] [PubMed]
- Siemienowicz M, Molan M, Doolan L. Massive anterior mediastinal mass causing cardiac compression. J Am Coll Cardiol 2010;56:e47. [Crossref] [PubMed]
- Minematsu N, Minato N, Kamohara K, et al. Complete removal of heart-compressing large mediastinal lipoma: a case report. J Cardiothorac Surg 2010;5:48. [Crossref] [PubMed]
- D'Cruz IA, Feghali N, Gross CM. Echocardiographic manifestations of mediastinal masses compressing or encroaching on the heart. Echocardiography 1994;11:523-33. [Crossref] [PubMed]
- Rankin S. [(18)F]2-fluoro-2-deoxy-D-glucose PET/CT in mediastinal masses. Cancer Imaging 2010;10 Spec no A:S156-60.
- Wignall OJ, Moskovic EC, Thway K, et al. Solitary fibrous tumors of the soft tissues: review of the imaging and clinical features with histopathologic correlation. AJR Am J Roentgenol 2010;195:W55-62. [Crossref] [PubMed]
- Andreini D, Pontone G, Dainese L, et al. Preoperative assessment of thymoma: evaluation of mediastinal arterial anatomy by cardiac multidetector computed tomography. J Thorac Imaging 2009;24:31-3. [Crossref] [PubMed]
- Rakovich G, Ferraro P, Therasse E, et al. Preoperative embolization in the management of a mediastinal paraganglioma. Ann Thorac Surg 2001;72:601-3. [Crossref] [PubMed]
- Baldó X, Sureda C, Gimferrer JM, et al. Primary mediastinal leiomyoma: an angiographic study and embolisation of the feeding vessels to improve the surgical approach. Eur J Cardiothorac Surg 1997;11:574-6. [Crossref] [PubMed]
- Morandi U, Stefani A, De Santis M, et al. Preoperative embolization in surgical treatment of mediastinal hemangiopericytoma. Ann Thorac Surg 2000;69:937-9. [Crossref] [PubMed]
- Ho MY, Fleischmann D, Forrester MD, et al. Coil embolization of a left circumflex feeder branch in a patient with a mediastinal paraganglioma. JACC Cardiovasc Interv 2011;4:1345-6. [Crossref] [PubMed]
- Vivas D, Ruiz-Mateos B, Franco E. Acute myocardial infarction secondary to direct myocardial infiltration by a malignant neoplasia. Eur Heart J 2010;31:2261. [Crossref] [PubMed]
- Qedra N, Kadry M, Ivanitskaia-Kühn E, et al. Solitary fibrous mediastinal tumor with coronary vascular supply: an unusual case. J Thorac Cardiovasc Surg 2010;139:e23-5. [Crossref] [PubMed]
- Allred JD, Mehta D, Courville KA, et al. Mediastinal mass with blood supply from the coronary arteries. Clin Cardiol 2009;32:E49. [Crossref] [PubMed]
- Beiras-Fernandez A, Uberfuhr P, Kaczmarek I, et al. Mediastinal pheochromocytoma with single coronary blood supply: a case report. Heart Surg Forum 2007;10:E196-8. [Crossref] [PubMed]
- Liu FY, Wang MQ, Fan QS, et al. Interventional embolization of giant thoracic tumors before surgical resection. Acta Radiol 2013;54:61-6. [Crossref] [PubMed]
- Lee SY, Lee JH, Hur GY, et al. Successful removal of a slowly growing mediastinal cavernous haemangioma after vascular embolization. Respirology 2006;11:493-5. [Crossref] [PubMed]
- Saraya T, Shimura C, Mikura S, et al. Huge mediastinal mass with SVC syndrome accompanying numerous chest wall collateral vessels. Intern Med 2008;47:1719-22. [Crossref] [PubMed]
- Stanford W, Jolles H, Ell S, et al. Superior vena cava obstruction: a venographic classification. AJR Am J Roentgenol 1987;148:259-62. [Crossref] [PubMed]
- Eren S, Karaman A, Okur A. The superior vena cava syndrome caused by malignant disease. Imaging with multi-detector row CT. Eur J Radiol 2006;59:93-103. [Crossref] [PubMed]
- Kesler KA, Rieger KM, Hammoud ZT, et al. A 25-year single institution experience with surgery for primary mediastinal nonseminomatous germ cell tumors. Ann Thorac Surg 2008;85:371-8. [Crossref] [PubMed]
- Koppitz H, Rockstroh JK, Schüller H, et al. State-of-the-art classification and multimodality treatment of malignant thymoma. Cancer Treat Rev 2012;38:540-8. [Crossref] [PubMed]
- Lucchi M, Van Schil P, Schmid R, et al. Thymectomy for thymoma and myasthenia gravis. A survey of current surgical practice in thymic disease amongst EACTS members. Interact Cardiovasc Thorac Surg 2012;14:765-70. [Crossref] [PubMed]
- Marchevsky A, Marx A, Ströbel P, et al. Policies and reporting guidelines for small biopsy specimens of mediastinal masses. J Thorac Oncol 2011;6:S1724-9. [Crossref] [PubMed]
- Assaad MW, Pantanowitz L, Otis CN. Diagnostic accuracy of image-guided percutaneous fine needle aspiration biopsy of the mediastinum. Diagn Cytopathol 2007;35:705-9. [Crossref] [PubMed]
- Moonim MT, Breen R, Gill-Barman B, et al. Diagnosis and subclassification of thymoma by minimally invasive fine needle aspiration directed by endobronchial ultrasound: a review and discussion of four cases. Cytopathology 2012;23:220-8. [Crossref] [PubMed]
- Tomaszek S, Wigle DA, Keshavjee S, et al. Thymomas: review of current clinical practice. Ann Thorac Surg 2009;87:1973-80. [Crossref] [PubMed]
- Ettinger DS, Akerley W, Bepler G, et al. Thymic malignancies. J Natl Compr Canc Netw 2010;8:1302-15. [PubMed]
- Wang Y, Zhang C, Liu J, et al. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res Treat 2012;131:357-69. [Crossref] [PubMed]
- Gillies RS, Middleton MR, Blesing C, et al. Metabolic response at repeat PET/CT predicts pathological response to neoadjuvant chemotherapy in oesophageal cancer. Eur Radiol 2012;22:2035-43. [Crossref] [PubMed]
- Shanmugan S, Arrangoiz R, Nitzkorski JR, et al. Predicting pathological response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer using 18FDG-PET/CT. Ann Surg Oncol 2012;19:2178-85. [Crossref] [PubMed]
- Gupta K, Pawaskar A, Basu S, et al. Potential role of FDG PET imaging in predicting metastatic potential and assessment of therapeutic response to neoadjuvant chemotherapy in Ewing sarcoma family of tumors. Clin Nucl Med 2011;36:973-7. [Crossref] [PubMed]
- Costa S, Terzano P, Santini D, et al. Neoadjuvant chemotherapy in cervical carcinoma: regulators of cell cycle, apoptosis, and proliferation as determinants of response to therapy and disease outcome. Am J Clin Pathol 2001;116:729-37. [Crossref] [PubMed]
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev 2012;5:CD005073. [PubMed]
- Jelic S, Sotiropoulos GC; ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v59-64. [Crossref] [PubMed]
- Blank RS, de Souza DG. Anesthetic management of patients with an anterior mediastinal mass: continuing professional development. Can J Anaesth 2011;58:853-9, 860-7. [Crossref] [PubMed]
- Anderson DM, Dimitrova GT, Awad H. Patient with posterior mediastinal mass requiring urgent cardiopulmonary bypass. Anesthesiology 2011;114:1488-93. [Crossref] [PubMed]
- Pullerits J, Holzman R. Anaesthesia for patients with mediastinal masses. Can J Anaesth 1989;36:681-8. [Crossref] [PubMed]
- Inoue M, Minami M, Shiono H, et al. Efficient clinical application of percutaneous cardiopulmonary support for perioperative management of a huge anterior mediastinal tumor. J Thorac Cardiovasc Surg 2006;131:755-6. [Crossref] [PubMed]
- Asai T. Emergency cardiopulmonary bypass in a patient with a mediastinal mass. Anaesthesia 2007;62:859-60. [Crossref] [PubMed]
- Demmy TL, Krasna MJ, Detterbeck FC, et al. Multicenter VATS experience with mediastinal tumors. Ann Thorac Surg 1998;66:187-92. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [Crossref] [PubMed]
- Beg RA, Naraghipour H, Kay EB, et al. Internal mammary retractor. Ann Thorac Surg 1985;39:286-7. [Crossref] [PubMed]
- Matsuura N, Ishikawa S, Misaki N, et al. A new application for the heart positioner in operations for mediastinal tumors. Ann Thorac Surg 2010;90:2063-4. [Crossref] [PubMed]
- Muralidaran A, Detterbeck FC, Boffa DJ, et al. Long-term survival after lung resection for non-small cell lung cancer with circulatory bypass: a systematic review. J Thorac Cardiovasc Surg 2011;142:1137-42. [Crossref] [PubMed]
- Bacha EA, Chapelier AR, Macchiarini P, et al. Surgery for invasive primary mediastinal tumors. Ann Thorac Surg 1998;66:234-9. [Crossref] [PubMed]
- Marulli G, Lucchi M, Margaritora S, et al. Surgical treatment of stage III thymic tumors: a multi-institutional review from four Italian centers. Eur J Cardiothorac Surg 2011;39:e1-7. [Crossref] [PubMed]
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007;356:1862-9. [Crossref] [PubMed]
- Xu X, Sun C, Zhang L, et al. A case of mediastinal seminoma presenting as superior vena cava syndrome. Intern Med 2012;51:1269-72. [Crossref] [PubMed]
- Dartevelle PG, Chapelier AR, Pastorino U, et al. Long-term follow-up after prosthetic replacement of the superior vena cava combined with resection of mediastinal-pulmonary malignant tumors. J Thorac Cardiovasc Surg 1991;102:259-65. [PubMed]
- Spaggiari L, Leo F, Veronesi G, et al. Superior vena cava resection for lung and mediastinal malignancies: a single-center experience with 70 cases. Ann Thorac Surg 2007;83:223-9; discussion 229-30. [Crossref] [PubMed]
- Leo F, Della Grazia L, Tullii M, et al. Hemodynamic instability during superior vena cava crossclamping: predictors, management, and clinical consequences. J Thorac Cardiovasc Surg 2007;133:1105-6. [Crossref] [PubMed]
- Sakamoto T, Duebener LF, Laussen PC, et al. Cerebral ischemia caused by obstructed superior vena cava cannula is detected by near-infrared spectroscopy. J Cardiothorac Vasc Anesth 2004;18:293-303. [Crossref] [PubMed]
- Ito R, Takita K, Mizunoya K, et al. Use of near-infrared spectroscopy in combination with monitoring of external jugular vein pressure for early detection of cerebral ischemia by unintentional superior vena cava obstruction. J Cardiothorac Vasc Anesth 2012;26:e27-8. [Crossref] [PubMed]
- Guarracino F. Cerebral monitoring during cardiovascular surgery. Curr Opin Anaesthesiol 2008;21:50-4. [Crossref] [PubMed]
- Spaggiari L, Thomas P, Magdeleinat P, et al. Superior vena cava resection with prosthetic replacement for non-small cell lung cancer: long-term results of a multicentric study. Eur J Cardiothorac Surg 2002;21:1080-6. [Crossref] [PubMed]
- Donahoo JS, Brawley RK, Gott VL. The heparin-coated vascular shunt for thoracic aortic and great vessel procedures: a ten-year experience. Ann Thorac Surg 1977;23:507-13. [Crossref] [PubMed]
- Gatta G, van der Zwan JM, Casali PG, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer 2011;47:2493-511. [Crossref] [PubMed]


