Comparison of single port versus multiport thoracoscopic segmentectomy
Introduction
Video-assisted thoracoscopic surgery (VATS) is becoming more popular for the treatment or diagnosis of lung disease. The wide acceptance of VATS is partly due to the reduction in postoperative pain and to a shorter recovery time compared to conventional thoracotomy (1). Recently, the treatment of choice for lung cancer has been changed to VATS for the early treatment of lung cancer in most centers (2). Anatomic lung resection, lobectomy or pneumonectomy, with the removal of the mediastinal lymph nodes are considered the treatment of choice and provide the best chance of survival in early lung cancer. Sublobar resection, segmentectomy or wedge resection, is considered alternative modalities for the treatment of clinical T1N0 lung cancer in high-risk patients with poor cardiopulmonary reserve who could not tolerate radical anatomic resection (3,4). VATS pulmonary segmentectomy became more popular and achieved through various additional techniques including preoperative localization (5) and the identification of the intersegmental plane (6,7).
More recently, the single-port VATS approach has advanced to perform many types of thoracic surgeries (8), including diagnostic procedures, minor and major lung anatomic resections (9), and the excision of mediastinal tumors. While it is not mandatory to use specialized instruments during single-port surgery, the handling of conventional endoscopic instruments through such limited port requires considerable surgical skills and operating time at an early learning period. Thus, this approach is currently limited as there are some negative perceptions regarding the need for specialized devices, the potential risk for complications, and the increased operating time and medical expenses associated with this procedure. Also, many thoracic surgeons believe that this approach has its limitation in oncologic clearance and outcome. There is still some controversy regarding adopting the single-port VATS approach in the thoracic surgical field.
VATS thoracic surgery, through a single-port, allows the direct vision to target structures and parallels the handling of instruments similar to open surgery (10), potentially with less intercostal pain (11) and comparable surgical outcomes (12) with an experienced operator providing more than just cosmetic results. Evidence continues to support single-port VATS as a feasible surgical option (13).
Single-port VATS pulmonary segmentectomy is considered a potentially advanced technique (12,14). Even with open thoracotomy or conventional VATS, pulmonary segmentectomy is still a technically demanding procedure compared to anatomic lobectomy. In addition to this, due to the location of the target lung segment, there is limited access to the segmental vessels and bronchus. Thus, a great concern for patient safety remains.
The authors report the surgical outcomes of single-port VATS segmentectomy in various lung diseases (15). We launched the single-port VATS major lung resection (lobectomy, bilobectomy, and pneumonectomy) in patients with lung malignancy from 2010 and single-port VATS segmentectomy from 2012 after a learning period from two port VATS (16). More recently, we adopted a 2-cm incision port in single-port VATS major lung resection for lung malignancy.
There has been relatively little information in the literature regarding the technical details and surgical outcomes of single-port VATS segmentectomy. The current study attempts to determine whether single-port VATS segmentectomy can play the alternative role in current minimal invasive thoracic surgery.
Materials and methods
Patients
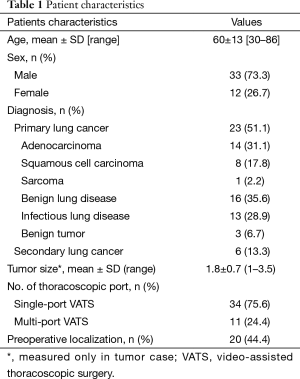
We retrospectively collected data on 45 patients who underwent a single-port (n=34) or multi-port (n=11) VATS pulmonary segmentectomy. The indication of pulmonary segmentectomy in our series included the peripherally located clinical T1N0M0 lung cancer with a lesion less than 2 cm in diameter with ground glass lesion showing a solid portion less than 50%, Inflammatory lung diseases which were resectable through segmentectomy instead of lobectomy to preserve normal lung parenchyma in patients with poor pulmonary reserve, or metastatic cancers or benign tumors were not available for wedge resection. VATS pulmonary segmentectomy was performed by multi-port approach (n=11, from 2006) before launching our single port VATS surgery from 2012. We compared the operating time, intraoperative event (conversion), mediastinal lymph node dissection, and postoperative outcome between single- port and multi-port VATS segmentectomy.
Operative procedure
Twenty patients underwent preoperative dual localization with hook-wire and radiocontrast or radioisotope under CT fluoroscopic guidance to identify the correct location of the lesion and achieve adequate intersegmental resection margins from the lesion (Figure 1). All localization procedures were performed 1–2 h before the operation. We used the intraoperative C-arm fluoroscopy to detect the radiocontrast injected around the target lesion before the division of the intersegmental plane.
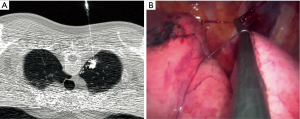
Anesthetic and surgical techniques for single-port VATS segmentectomy were not significantly different from those of single-port VATS lobectomy. The operator was always on the right side of the patient and positioned to the lateral decubitus, and made a 2 to 4 cm single-port incision at the 5th intercostal space on the anterior or posterior axillary line according to the location of the lesion. We always applied wound protector on the port to achieve better instrumental performance. We used a 5-mm thoracoscope in most of the cases. We used a 5-mm diameter articulating endoscopic device, a conventional endoscopic device with the shaft shortened, curved tip electrocautery, flexible curved-tip endostaplers, and interlocking vascular clips for branches of pulmonary segmental vessels if staplers were not adequate during single-port VATS segmentectomy. We used a 35-mm vascular stapler for the division of segmental vessels, sometimes by the guidance of a soft drain.
Detailed procedures and sequences of single-port VATS segmentectomy by pulmonary segment were as follows. During segmentectomy for the upper lobar segment, we started the dissection of the interlobar fissure to expose the pulmonary segmental artery. After exposure of the pulmonary segmental artery for target pulmonary segment, segmental artery branches were divided with a flexible curved tip vascular stapler or by double clipping the interlocking vascular clips (Figure 2A). After traction of the lung to posteriorly, the pulmonary vein could be dissected, and segmental branches of the pulmonary vein exposed by further dissection of the mediastinal pleura. Isolated segmental pulmonary veins could be divided after careful dissection of the posterior vein wall to avoid injury to the apical branches of the pulmonary artery to the upper lobe (Figure 2B). We divided the apical segmental artery before the vein division to facilitate the passing of the stapler if there was tension or difficulty in stapling the segmental vein. After releasing the perivascular and peribronchial tissues and lymph nodes, the segmental bronchus was isolated and divided by the stapler (Figure 2C). Before the division of the segmental bronchus, we performed intraoperative fiberoptic bronchoscopy for the correct identification of the target segment (Figure 2D). We used inflation and deflation techniques before stapling the segmental bronchus for the delineation of the intersegmental plane. The anesthesiologist administered a 2 kg/cm2 pressure of jet ventilation (Figure 2E,F). After stapling the segmental bronchus, we divided the intersegmental plane with the guidance of the C-arm fluoroscope to achieve adequate intersegmental resection margins from the target lesion (Figure 2G).
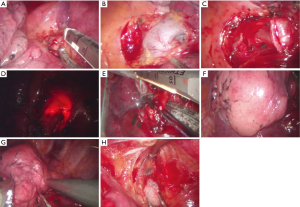
For single-port VATS segmentectomy of the lower lobe segment and superior or basal segmentectomy of the lower lobe, the procedure started with the release of the inferior pulmonary ligament to expose the inferior pulmonary segmental vein. We dissected the interlobar segmental pulmonary artery by fissure exposure. The vein dissection was the next step to expose the segmental bronchus to the lower lobe. Ideally, the perivascular and peribronchial between the segmental artery and bronchus should be removed to isolate the target segmental bronchus. Finally, we divided the segmental bronchus and intersegmental plane using the same technique we used to divide the upper lobe segment. We removed the segmentectomy specimen by a protective endo-bag through the single-port.
We performed complete lymph node dissection including upper and lower mediastinal, subcarinal, and lobe-specific lymph nodes for occult metastasis. In some cases, we performed dissection around specific lobes with sampling in non-specific lobes. In our surgeries on left thorax, para-aortic and subaortic lymph nodes were dissected routinely with the endoscopic node grasper (Figure 2H). We placed a 20- or 24-French chest tube and intrapleural catheter for continuous analgesic injection pump through the single-port. The apico-posterior segmentectomy procedure is available in the Figure 3.

Postoperative courses
We removed the chest drains according to our criteria; drain amount less than one-third of the patient’s body weight per day, no air leak, and no pneumothorax on chest image. The patient was discharged the day after the removal of the chest drain if there were no postoperative complications.
Results
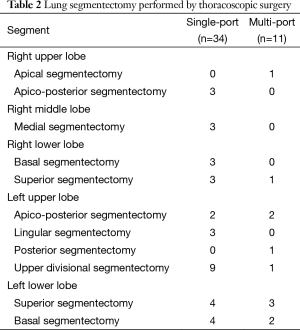
From March 2006 through October 2015, 45 patients underwent VATS segmentectomy in our institution for lung malignancy or benign lung disease. Among these patients, 34 (76.5%) underwent a segmentectomy performed by single-port VATS from 2012. Twenty-four (51.1%) had primary lung malignancies; indications for pulmonary segmentectomy included peripherally located clinical T1N0 lung cancer less than 2 cm in diameter showing less than 50% solid portion. In addition to these patients, we included six secondary lung cancer (13.3%) patients and 16 benign lung diseases (35.6%) confined to specific pulmonary segment resectable through segmentectomy in our study. The mean tumor size was 1.8±0.7 cm (Table 1). The age of patients ranged from 30 to 86 years (mean 60±13 years), our study population was primarily male 73.3% (n=33). Segmentectomy for the upper divisional segment of the upper lobe, the superior segment of the lower lobe, the lingular segment, and the basal segment were the most common procedures (Table 2).
Full table
Full table
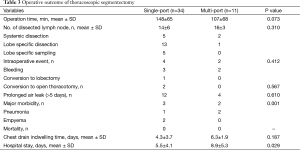
The operation time in single-port VATS segmentectomy (148±65 min) was longer compared to multi-port VATS segmentectomy (107±68 min). However, this difference was not significant (P=0.073). The number of resected lymph nodes during VATS segmentectomy (n=24) was higher (P=0.031) in the multi-port VATS group compared to the single-port VATS group; these occurred in a relatively small population (n=3). In the single-port VATS group, dissection of lymph nodes around a specific lobe was performed in 13 patients and complete systemic dissection was performed in five patients. Bleeding was the most common event reported during VATS segmentectomy (three in single-port and two in multi-port) which we controlled without conversion in most cases. Conversion to mini-thoracotomy occurred in two patients in the single-port VATS segmentectomy group. There was one conversion to lobectomy as we failed to find the lung lesion in the segmentectomy specimen. There was no lymph node metastasis in patients with malignancy at pathologic results. Prolonged air leak (>5 days) was the most common minor postoperative event in our study population. However, there were no significant differences between groups (P=0.610). Three patients developed postoperative pneumonia (one in single-port and two in multi-port) which resolved in all patients with antibiotic treatment. Two patients developed postoperative empyema in the single-port VATS segmentectomy group. There was no postoperative mortality within 30 days. Indwelling time of the chest drain was unchanged in the single-port VATS segmentectomy group. However, the hospital stay was decreased in the single-port VATS segmentectomy group (5.5±4.1 days, P=0.029) (Table 3).
Full table
Discussion
Our results indicate that single-port VATS is a feasible option and should be a VATS alternative for pulmonary segmentectomy in early lung cancer. In our study, there were a small number of multi-port VATS segmentectomies because sublobar resection has not been favorable in the surgical treatment of lung cancer; it has a higher rate of local recurrence and poor survival compared to those undergoing a lobectomy. However, recently, it has been reported that VATS segmentectomy achieves excellent oncologic results compared with thoracotomy in early lung cancer (<2 cm in size, typically adenocarcinoma) with low morbidity (~10%) and mortality (18). Also, in our institution, we have been changing our surgical strategy to minimal lung resection with a minimal incision in early stage non-small lung cancers and other lung malignancies for better lung function. With our single-port VATS experiences, the authors report the possibility of performing single-port VATS segmentectomy with proper localization techniques in patients with various lung diseases without expensive special devices. However, there remains little information on the surgical outcomes of single-port VATS compared with multi-port VATS. Future prospective cohort studies are needed to address the surgical outcomes of single-port VATS.
Nonetheless, our study reported better postoperative outcomes (morbidity and hospital stay) associated with single-port VATS. It is also evident that more operating time in the early period is needed when operating a single-port VATS compared to multi-port VATS. Operation time decreases as surgeons gain more experience; thus, this shouldn’t be an issue with experienced surgeons for single port segmentectomy. In our study, the results of mediastinal lymph node dissection in lung malignancy were no worse than patients undergoing single-port VATS. The need for complete lymph node dissection is not clear in sublobar resection and should be studied in VATS (19). An advantage of the single-port VATS approach is the direct endoscopic view that allows the surgeon a target similar to that of open thoracotomy that may be helpful in the dissection of the segmental vessel. The disadvantages of the single-port VATS are that this procedure is still technically difficult to conduct in the early learning period and might not be safe if performed by an unexperienced surgeon.
Technically, the steps performed in single-port VATS segmentectomy are no different from those of the multi-port VATS. The surgeon should consider the operative plan with preoperative CT and PET scan before launching the single-port VATS segmentectomy. The most common and easiest lung segment is the superior segment and lingular segment on both lower lobes. The upper division (trisegment) of the left upper lobe and the composite basilar segment of either lower lobe are difficult segments to access for segmentectomy. Apical and/or posterior segmentectomy of the right upper lobe is not indicated in the presence of emphysema in the upper lobes (20). Adequate port placement is considered based on the target lung segments. We favor the 5th intercostal space at the anterior or posterior axillary line according to the tumor location. Comprehensive understanding of lung segmental anatomy should be carried out to dissect and divide the correct segmental vessels. Technically, there are no limits of single-port VATS segmentectomy according to the lung segments. After the division of the segmental vessels, the segmental bronchus can be identified, and the peribronchial tissue released for safe stapling. Before the division of the segmental bronchus, the surgeon should confirm the correct bronchus by lung inflation after clamping the bronchus or inspecting it via intraoperative bronchoscopy. To divide the intersegmental plane with adequate resection margins (more than 2 cm from the lesion or more than the tumor size), a preoperative localization or a jet inflation technique, or intravenous injection of isocyanine green could help to delineate the intersegmental imaginary fissure.
In summary, currently, the single-port VATS approach may not be popular in the thoracic surgical field as there are many technical limitations to performing advanced VATS procedures. Future studies are needed to ascertain the acceptable long-term outcomes and patient safety of the VATS. The single-port VATS approach applies to most thoracic surgeries if indicated for pulmonary segmentectomy. It appears that this surgical approach might play an important role in updating minimal resection with minimal incision.
Acknowledgements
Funding: This work was supported by a Grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A121074) and a National Research Foundation of Korea (NRF) Grant funded by the Ministry of Education, Science and Technology (No. NRF-2015R1A2A2A04005760).
Footnote
Conflicts of Interest: This paper is an invited article for ASPVS 2016. Speaker is Prof. Hyun Koo Kim as 4th ASPVS faculty member. The other authors have no conflicts of interest to declare.
References
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Flores RM, Alam N. Video-assisted thoracic surgery lobectomy (VATS), open thoracotomy, and the robot for lung cancer. Ann Thorac Surg 2008;85:S710-5. [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 1997;114:347-53. [PubMed]
- Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 2001;71:956-60; discussion 961. [PubMed]
- Doo KW, Yong HS, Kim HK, et al. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol 2015;22:331-7. [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [PubMed]
- Zhang Z, Liao Y, Ai B, et al. Methylene blue staining: a new technique for identifying intersegmental planes in anatomic segmentectomy. Ann Thorac Surg 2015;99:238-42. [PubMed]
- Rocco G, Martucci N, La Manna C, et al. Ten-year experience on 644 patients undergoing single-port (uniportal) video-assisted thoracoscopic surgery. Ann Thorac Surg 2013;96:434-8. [PubMed]
- Ng CS, Kim HK, Wong RH, et al. Single-Port Video-Assisted Thoracoscopic Major Lung Resections: Experience with 150 Consecutive Cases. Thorac Cardiovasc Surg 2015. [Epub ahead of print]. [PubMed]
- Bertolaccini L, Rocco G, Viti A, et al. Geometrical characteristics of uniportal VATS. J Thorac Dis 2013;5 Suppl 3:S214-6. [PubMed]
- Tamura M, Shimizu Y, Hashizume Y. Pain following thoracoscopic surgery: retrospective analysis between single-incision and three-port video-assisted thoracoscopic surgery. J Cardiothorac Surg 2013;8:153. [PubMed]
- Wang BY, Tu CC, Liu CY, et al. Single-incision thoracoscopic lobectomy and segmentectomy with radical lymph node dissection. Ann Thorac Surg 2013;96:977-82. [PubMed]
- Zeltsman D. Current readings: Redefining minimally invasive: uniportal video-assisted thoracic surgery. Semin Thorac Cardiovasc Surg 2014;26:249-54. [PubMed]
- Gonzalez-Rivas D. Single incision video-assisted thoracoscopic anatomic segmentectomy. Ann Cardiothorac Surg 2014;3:204-7. [PubMed]
- Han KN, Kim HK, Lee HJ, et al. Single-port video-assisted thoracoscopic pulmonary segmentectomy: a report on 30 cases†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i42-i47. [PubMed]
- Kim HK, Choi YH. The feasibility of single-incision video-assisted thoracoscopic major pulmonary resection performed by surgeons experienced with a two-incision technique. Interact Cardiovasc Thorac Surg 2015;20:310-5. [PubMed]
- Han KN, Kim HK, Choi YH. Single-port VATS segmentectomy: left upper lobe apico-posterior segmentectomy. Asvide 2016;3:074. Available online: http://www.asvide.com/articles/827
- Okada M. Radical sublobar resection for small-diameter lung cancers. Thorac Surg Clin 2013;23:301-11. [PubMed]
- Darling GE. Current status of mediastinal lymph node dissection versus sampling in non-small cell lung cancer. Thorac Surg Clin 2013;23:349-56. [PubMed]
- Swanson SJ. Segmentectomy for lung cancer. Semin Thorac Cardiovasc Surg 2010;22:244-9. [PubMed]


