Airway anastomosis for lung transplantation
Introduction
Lung transplantation (LT) is currently considered the only viable option for a selected group of patients with end stage pulmonary disease not responding to medical or surgical therapies and with a life expectancy of less than 2 years. The most common indications for LT include four groups of diseases: obstructive, restrictive, septic and vascular; survival varies according to the underlying disorder, with better results for cystic fibrosis and emphysema and worse for idiopathic pulmonary fibrosis. During the last 20 years the technical aspects of the procedure, organ preservation, perioperative management and immunosuppression have been dramatically improved.
Since the early days of LT, healing of the airway anastomosis has been considered the Achilles’ heel limiting survival (1-3). A number of experimental studies have been performed to understand the causes of healing impairment and to reduce the risk of catastrophic events related to airway complications (4,5). The development of the bilateral sequential technique with separate bronchial anastomoses has been somehow forced by the high rate of anastomotic tracheal dehiscence (25%) leading to fatal events (6,7) reported for the en-bloc procedure with tracheal anastomosis. Furthermore, LT represents an exception in the transplantation world: in fact, the lung is the only organ in which the arterial systemic blood supply (bronchial arteries) is not routinely restored during the transplant and a network of bronchial circulation around the anastomosis is detectable only after 4 weeks (8). This detail may explain the frailty of the anastomotic site and why different factors including donor management, high—dose steroids administration, immunosuppression therapy, surgical technique and perioperative management can affect its correct healing.
Historical background
The current knowledge of the process of airway healing after LT is based on experimental studies performed during 1980s by the Toronto Lung Transplant Group on auto-transplanted lungs in dogs. Initially, they focused on the effects of steroids and azathioprine (the only immunosuppressive drugs used at that time) on the breaking strength of bronchial anastomosis; they reported that only steroids were responsible for the impaired healing while azathioprine had no effects (3). The introduction in the clinical practice of cyclosporine A (CSA) allowed to dramatically decrease the rate of airway complications in the same animal model. These data were confirmed at scanning electron microscopy, showing normal collagen formation at the anastomotic site in animals receiving CSA (4). These studies contributed to reduce the use of steroids before and after LT to avoid impairing of the healing process. However, further studies demonstrated that the administration of steroids plays an important role to prevent rejection and, at ameliorate the patency of microcirculation in case of reperfusion injury (9,10). Low dose steroids also contribute to improve healing of the anastomosis in a non-transplant setting (11). For these reasons they are still included in the immunosuppressive regimen. However, their dose should be reduced as much as possible before the transplant (12).
The effects of the interruption of the bronchial circulation have long been debated. Early studies performed in 1960s showed that if the bronchial vessels are not anastomosed a higher rate of bronchial complications is observed (13,14). However, subsequent studies showed that a fine network of bronchial circulation is detectable starting from the fourth week (8) and that an early network of vessels surrounding the anastomosis is already present after 12–14 days (15). This data confirms previous reports stressing that the first two postoperative weeks are crucial to prevent airway complications (16). Based on these reports, the Toronto Group proposed to buttress the anastomosis with an omental pedicle flap to reduce the early ischemic time and enhance the microcirculation (17,18). They demonstrated that with such technique, after 4 days a network of multiple capillaries originating from the omentum surrounds the bronchus and supports healing. Although this technique has initially met a large consensus, it has been progressively abandoned in favor of new and technically easier strategies to wrap the anastomosis as the use of the intercostal muscle (19,20) or the peribronchial tissue (21).
Evolution of technical details
Since bronchial anastomosis complications can be catastrophic and significantly affect outcome, the technical aspects of suturing have been repeatedly modified and simplified since the early days. The bronchial anastomosis was initially performed after the vascular anastomoses due to the lateral decubitus of the patient on the operatory table; the cartilaginous portion was completed first with interrupted absorbable sutures, followed by the membranous portion. At the end, the omentum was transposed in the chest and wrapped around the suture line (22). The supine position on the operatory table forced to perform the bronchial anastomosis first, starting from the membranous portion.
Due to the peculiarity of the airway vascular support (low pressure circulation from the pulmonary arteries and systemic pressure circulation from bronchial arteries; both divided at time of transplantation), the length of the donor bronchus has been historically considered crucial to prevent airway complications. The donor bronchus is usually transected no more than one or two rings above the lobar carina to minimize the area of ischemia (23). More recently, several reports suggested that an even shorter length of donor bronchus (close to the lobar carina) might further reduce bronchial ischemia (24-26). This modification significantly contributed to decrease the rate of airway complications, independently from the surgical technique used to perform the anastomosis. However, the excessive shortness of the donor bronchus could create problems to treat major complications in case they occur; in fact, in such situation, mechanical dilation or stent placement might be difficult and sleeve lobectomy or redo transplantation might become the only available options (25).
Bronchial artery revascularization with microsurgery techniques has been proposed to improve healing (27). Although this approach allows full restoration of the bronchial circulation, the technical difficulty and the additional operative time have limited worldwide spreading.
The surgical technique for bronchial anastomosis has been repeatedly modified and even now there are differences between centers. Even the type of suture material is still debated (absorbable vs. non-absorbable). The classic technique proposed by the Toronto group was an end-to-end anastomosis with an absorbable 4/0 running suture on the membranous part and single or figure-of-eight stiches for cartilaginous wall (23). Briefly, a silk traction suture or an Ellis clamp is placed at the midpoint of the cartilaginous portion of the recipient airway to retract the bronchus from the mediastinum. The first step is to approximate the donor and recipient posterior peribronchial tissue followed by a running suture of the membranous portion. The cartilaginous part is sutured with single or figure-of eight stitches, progressively adjusting the mismatch between the stumps and the silk stitch is removed. After completion of the anastomosis, the suture on the posterior peribronchial tissue is continued anteriorly covering the bronchus. This approach has represented, and still represents at several transplant centers, the gold standard. However, some limitations compared to a complete running suture (membranous plus cartilaginous portions) as more time required to perform it, and inflammation caused by multiple stitches that may potentially affect the correct healing have been reported (28,29). For these reasons some authors prefer an end-to-end running technique with an absorbable 4/0 monofilament suture. Although coverage of the anastomosis is usually considered mandatory, at some centers it is not performed at all (Vienna Lung Transplant Center), with equally good results (28). The rate of airway complications is similar with either technique (Table 1); furthermore both of them allow easily overcoming of the potential size mismatch between donor and recipient airway.

Full table
Telescoping anastomosis with the intussusception of the donor bronchus into the recipient airway has gained widespread consensus in the 1990s to solve the problem of size mismatch and to improve the tightness of anastomosis (30,31); only the cartilaginous part of the bronchus is intussuscepted. However, due to the higher incidence of anastomotic complications, this technique has progressively been relegated to those cases with a natural tendency towards intussusception (28,32).
The technique of bronchial anastomosis in case of pediatric or lobar transplantation (cadaveric or living related) is similar, although the size of the suture is smaller (5/0). In case of living related lobar transplantation, the dissection of the donor bronchus should be minimized to preserve backward blood supply. On right side, the middle lobe bronchus is identified and the incision goes obliquely from above the superior segment bronchus of the lower lobe to just below the middle lobe bronchus; on left side the lower lobe bronchus is transected tangentially above the superior segment of the lower lobe (33).
Risk factors for airway complications after LT
Several risk factors have been considered in the development of airway complications after LT: ischemia, impaired organ preservation, rejection and infection. Prolonged mechanical ventilation of both donor and recipient has been considered to play a role with different mechanisms: by causing a persistent inflammation status and an higher risk of infection in the donor and by determining a barotrauma on the anastomosis in the recipient; furthermore the need of prolonged mechanical ventilation after LT may be a sign of graft failure as a result of prolonged ischemia (25,34). Thus, patients should be extubated as soon as possible (35)
Adequate organ preservation is crucial. The use of low potassium dextrane solutions associated to the administration of prostaglandins to increase the microcirculation flow (36) and the association of retrograde perfusion (37) have contributed to decreased the rate of airway complications; furthermore, limiting the cold ischemic time within 6-8 hours should minimize the risk of injury (38).
Acute rejection has been identified as an independent risk factor for airway complications by causing acute inflammation, submucosal edema and increased vascular resistance with subsequent reduction of graft perfusion (36). Administration of low dose steroids may ameliorate the microcirculation by reducing edema with improvement of perfusion at the anastomotic site; thus, optimizing immunosuppression is crucial.
A strong association between airway complications and Aspergillus infection has been reported (39). Fungal infections are relatively frequent in transplant patients (40). The simultaneous presence of anastomotic necrosis and Aspergillus is correlated with a higher risk of late bronchial complications compared to the presence of necrosis alone. Broncho-arterial fistula has also been reported. An aggressive antifungal therapy should be immediately started even in asymptomatic patients.
Bronchial complications
The incidence of bronchial complications ranges between 7% and 18% with a mortality between 2% and 5% (24,34,41). Early and late complications include bleeding, necrosis, dehiscence, granulations, stenosis and malacia (42). Although several classifications of bronchial healing have been proposed, none has been worldwide accepted. The Couraud grading system based on bronchoscopic surveillance at the 15th postoperative day is well known and it seems to show a correlation with the subsequent onset of airway complications (43). Anastomotic healing is classified as follows:
- Grade 1: complete circumferential primary mucosal healing;
- Grade 2A: complete circumferential primary healing of the airway wall without necrosis and with partial primary mucosal healing;
- Grade 2B: complete circumferential primary healing of the airway wall without necrosis but with no primary mucosal healing;
- Grade 3A: limited focal necrosis (extending less than 5 mm from the anastomotic line);
- Grade 3B: extensive necrosis.
The development of anastomotic necrosis and dehiscence is related to an ischemic injury and the severity of this complication goes from a focal superficial lesion to extensive necrosis of the bronchial wall that may determine catastrophic consequences with high mortality. These events can be detected in asymptomatic patients during bronchoscopic surveillance or they can be highlighted with radiological studies (computed tomography with multiplanar reconstructions) in patients showing clinical manifestations like fever, cough, dyspnea, prolonged air leaks, pneumothorax, pneumomediastinum with subcutaneous emphysema and mediastinitis with sepsis. When healing, these complications may lead to granulation, stenosis or bronchomalacia. Treatment is based on the severity of the problem, ranging from a conservative approach or minimally invasive treatment for mild lesions to more aggressive therapeutic options including reconstructive surgery, pneumonectomy or retransplantation. In case of a very limited and asymptomatic dehiscence a “wait and see policy” with continuous bronchoscopic surveillance and bronchial debridement can be the first approach; the instillation of glues or sealants has been often reported (44) (Figure 1). Stent placement (silicone or covered expandable metallic) is the following step. However, in difficult cases, surgery may be required: direct suture, sleeve resection, pneumonectomy and re- transplantation have been reported, although they carry significant morbidity and mortality (45).
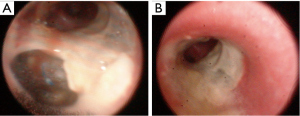
The onset of granuloma usually requires mechanical debridement or laser ablation (46,47). Prevention of recurrence, occurring in 10–50% of cases, includes injection of Anti-fibroblast and anti-inflammatory agents in the bronchial wall to avoid fibroblast proliferation and formation of granulation tissue. Although several drugs have been used, the results are still controversial and the exact dose is not well established yet (47).
The most frequent bronchial complication following LT is stenosis occurring either at the level of the anastomosis or more distally. It is usually related to ischemia and impaired local microcirculation; however, a diffuse peripheral stricture might be a manifestation of cellular rejection. Patients with bronchial stenosis can be absolutely asymptomatic or present with dyspnea, cough and recurrent pulmonary infections; pulmonary function tests (PFTs) may show a reduction in the forced expiratory volume in 1 second (FEV1). Treatment includes mechanical dilation with the rigid bronchoscope or other instruments (48), balloon bronchoplasty and stenting. The choice of the stent should be evaluated on a case-by-case basis. Silicone stents are usually easy to deploy, they can be removed even after a long period of time and the cost is low; however, there are some disadvantages including the need of constant nebulization to promote airway clearance and the challenging placement in case of a tortuous airway. In this case the use of metallic stents might be helpful (49); however, the complications related to these devices are formation of granulation tissue, airway rupture due to erosion and extreme difficulty in case removal is required.
Malacia is a condition in which the airway tends to collapse during breathing or with cough and it is generally due to ischemia, infection or altered response of the bronchial wall to immunosuppression. Symptoms are dyspnea and stridor mostly evident during exercise, cough and wheezing; PFTs show a marked reduction of all dynamic volumes [FEV1, forced expiratory flow of 25% to 75% (FEF25-75) and peak expiratory flow (PEF)]; bronchoscopy allows to confirm the diagnosis. Stenting is usually required.
Overall, early diagnosis of bronchial complications and their correct management are crucial to achieve satisfactory results and a better survival after LT.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Hardy JD, Webb WR, Dalton ML Jr, et al. Lung homotransplantation in man. JAMA 1963;186:1065-74. [PubMed]
- Wildevuur CR, Benfield JR. A review of 23 human lung transplantations by 20 surgeons. Ann Thorac Surg 1970;9:489-515. [PubMed]
- Cooper JD. Herbert Sloan lecture. Lung transplantation. Ann Thorac Surg 1989;47:28-44. [PubMed]
- Lima O, Cooper JD, Peters WJ, et al. Effects of methylprednisolone and azathioprine on bronchial healing following lung autotransplantation. J Thorac Cardiovasc Surg 1981;82:211-5. [PubMed]
- Goldberg M, Lima O, Morgan E, et al. A comparison between cyclosporin A and methylprednisolone plus azathioprine on bronchial healing following canine lung autotransplantation. J Thorac Cardiovasc Surg 1983;85:821-6. [PubMed]
- Patterson GA, Cooper JD, Goldman B, et al. Technique of successful clinical double-lung transplantation. Ann Thorac Surg 1988;45:626-33. [PubMed]
- Patterson GA, Todd TR, Cooper JD, et al. Airway complications after double lung transplantation. Toronto Lung Transplant Group. J Thorac Cardiovasc Surg 1990;99:14-20; discussion 20-1. [PubMed]
- Pearson FG, Goldberg M, Stone RM, et al. Bronchial arterial circulation restored after reimplantation of canine lung. Can J Surg 1970;13:243-50. [PubMed]
- Pinsker KL, Veith FJ, Kamholz SL, et al. Influence of bronchial circulation and corticosteroid therapy on bronchial anastomotic healing. J Thorac Cardiovasc Surg 1984;87:439-44. [PubMed]
- Novick RJ, Menkis AH, McKenzie FN, et al. The safety of low-dose prednisone before and immediately after heart-lung transplantation. Ann Thorac Surg 1991;51:642-5. [PubMed]
- Rendina EA, Venuta F, Ricci C. Effects of low-dose steroids on bronchial healing after sleeve resection. A clinical study. J Thorac Cardiovasc Surg 1992;104:888-91. [PubMed]
- Venuta F, Rendina EA, Ciriaco P, et al. Efficacy of cyclosporine to reduce steroids in patients with idiopathic pulmonary fibrosis before lung transplantation. J Heart Lung Transplant 1993;12:909-14. [PubMed]
- Stone RM, Ginsberg RJ, Colapinto RF, et al. Bronchial artery regeneration after radical hilar stripping. Surg Forum 1966;17:109-10. [PubMed]
- Mills NL, Boyd AD, Gheranpong C. The significance of bronchial circulation in lung transplantation. J Thorac Cardiovasc Surg 1970;60:866-78. [PubMed]
- Siegelman SS, Hagstrom JW, Koerner SK, et al. Restoration of bronchial artery circulation after canine lung allotransplantation. J Thorac Cardiovasc Surg 1977;73:792-5. [PubMed]
- Rabinovich JJ. Re-establishment of bronchial arteries after experimental lung lobe autotransplantation. J Thorac Cardiovasc Surg 1972;64:119-26. [PubMed]
- Lima O, Goldberg M, Peters WJ, et al. Bronchial omentopexy in canine lung transplantation. J Thorac Cardiovasc Surg 1982;83:418-21. [PubMed]
- Morgan E, Lima O, Goldberg M, et al. Successful revascularization of totally ischemic bronchial autografts with omental pedicle flaps in dogs. J Thorac Cardiovasc Surg 1982;84:204-10. [PubMed]
- Rendina EA, Venuta F, Ricci P, et al. Protection and revascularization of bronchial anastomoses by the intercostal pedicle flap. J Thorac Cardiovasc Surg 1994;107:1251-4. [PubMed]
- Quattrucci S, Rolla M, Cimino G, et al. Lung transplantation for cystic fibrosis: 6-year follow-up. J Cyst Fibros 2005;4:107-14. [PubMed]
- Miller JD, DeHoyos A. An evaluation of the role of omentopexy and of early perioperative corticosteroid administration in clinical lung transplantation. The University of Toronto and Washington University Lung Transplant Programs. J Thorac Cardiovasc Surg 1993;105:247-52. [PubMed]
- Cooper JD, Pearson FG, Patterson GA, et al. Technique of successful lung transplantation in humans. J Thorac Cardiovasc Surg 1987;93:173-81. [PubMed]
- Lau CL, Patterson GA. Technical considerations in lung transplantation. Chest Surg Clin N Am 2003;13:463-83. [PubMed]
- Weder W, Inci I, Korom S, et al. Airway complications after lung transplantation: risk factors, prevention and outcome. Eur J Cardiothorac Surg 2009;35:293-8; discussion 298. [PubMed]
- FitzSullivan E, Gries CJ, Phelan P, et al. Reduction in airway complications after lung transplantation with novel anastomotic technique. Ann Thorac Surg 2011;92:309-15. [PubMed]
- van Berkel V, Guthrie TJ, Puri V, et al. Impact of anastomotic techniques on airway complications after lung transplant. Ann Thorac Surg 2011;92:316-20; discussion 320-1. [PubMed]
- Nørgaard MA, Olsen PS, Svendsen UG, et al. Revascularization of the bronchial arteries in lung transplantation: an overview. Ann Thorac Surg 1996;62:1215-21. [PubMed]
- Aigner C, Jaksch P, Seebacher G, et al. Single running suture--the new standard technique for bronchial anastomoses in lung transplantation. Eur J Cardiothorac Surg 2003;23:488-93. [PubMed]
- Puri V, Patterson GA. Adult lung transplantation: technical considerations. Semin Thorac Cardiovasc Surg 2008;20:152-64. [PubMed]
- Kshettry VR, Shumway SJ, Gauthier RL, et al. Technique of single-lung transplantation. Ann Thorac Surg 1993;55:1019-21. [PubMed]
- Schröder C, Scholl F, Daon E, et al. A modified bronchial anastomosis technique for lung transplantation. Ann Thorac Surg 2003;75:1697-704. [PubMed]
- Garfein ES, Ginsberg ME, Gorenstein L, et al. Superiority of end-to-end versus telescoped bronchial anastomosis in single lung transplantation for pulmonary emphysema. J Thorac Cardiovasc Surg 2001;121:149-54. [PubMed]
- Bowdish ME, Barr ML, Starnes VA. Living lobar transplantation. Chest Surg Clin N Am 2003;13:505-24. [PubMed]
- Van De Wauwer C, Van Raemdonck D, Verleden GM, et al. Risk factors for airway complications within the first year after lung transplantation. Eur J Cardiothorac Surg 2007;31:703-10. [PubMed]
- Rocca GD, Coccia C, Costa GM, et al. Is very early extubation after lung transplantation feasible? J Cardiothorac Vasc Anesth 2003;17:29-35. [PubMed]
- Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc 2009;6:79-93. [PubMed]
- Venuta F, Rendina EA, Bufi M, et al. Preimplantation retrograde pneumoplegia in clinical lung transplantation. J Thorac Cardiovasc Surg 1999;118:107-14. [PubMed]
- Denlinger CE, Meyers BF. Update on lung transplantation for emphysema. Thorac Surg Clin 2009;19:275-83. [PubMed]
- Herrera JM, McNeil KD, Higgins RS, et al. Airway complications after lung transplantation: treatment and long-term outcome. Ann Thorac Surg 2001;71:989-93; discussion 993-4. [PubMed]
- Pugliese F, Ruberto F, Cappannoli A, et al. Incidence of fungal infections in a solid organ recipients dedicated intensive care unit. Transplant Proc 2007;39:2005-7. [PubMed]
- Shennib H, Massard G. Airway complications in lung transplantation. Ann Thorac Surg 1994;57:506-11. [PubMed]
- Kshettry VR, Kroshus TJ, Hertz MI, et al. Early and late airway complications after lung transplantation: incidence and management. Ann Thorac Surg 1997;63:1576-83. [PubMed]
- Couraud L, Nashef SA, Nicolini P, et al. Classification of airway anastomotic healing. Eur J Cardiothorac Surg 1992;6:496-7. [PubMed]
- Chhajed PN, Malouf MA, Tamm M, et al. Interventional bronchoscopy for the management of airway complications following lung transplantation. Chest 2001;120:1894-9. [PubMed]
- Puchalski J, Lee HJ, Sterman DH. Airway complications following lung transplantation. Clin Chest Med 2011;32:357-66. [PubMed]
- Sonett JR, Keenan RJ, Ferson PF, et al. Endobronchial management of benign, malignant, and lung transplantation airway stenoses. Ann Thorac Surg 1995;59:1417-22. [PubMed]
- Penafiel A, Lee P, Hsu A, et al. Topical mitomycin-C for obstructing endobronchial granuloma. Ann Thorac Surg 2006;82:e22-3. [PubMed]
- Venuta F, Rendina EA, De Giacomo T, et al. Operative endoscopy of the airway with the old-fashioned esophageal dilators. Ann Thorac Surg 2005;79:718-9. [PubMed]
- Anile M, Venuta F, Diso D, et al. Treatment of complex airway lesions after lung transplantation with self-expandable nitinol stents: early experience. Transplant Proc 2010;42:1279-80. [PubMed]


