A modified Sequential Organ Failure Assessment score using the Richmond Agitation-Sedation Scale in critically ill patients
In the January 2016 issue of Critical Care Medicine, Vasilevskis et al. (1) reported the validation study of a modified Sequential Organ Failure Assessment (SOFA) score using the Richmond Agitation-Sedation Scale (RASS) (2) instead of the Glasgow Coma Scale (GCS) for the neurological component. As explained by the authors, the SOFA score, first shown to describe multiple organ failure in patients with sepsis, is now widely used for risk stratification in a wide panel of ICU patients. Unfortunately, the GCS component is subject to implementation difficulties for some patients, such as those who are intubated, and is not highly reliable when assessing neurologic disorders such as delirium and agitation.
Vasilevskis and colleagues (1) assessed the validity of the RASS-based SOFA scoring system (Table 1) within the BRAIN-ICU study population, a prospective cohort study of critically ill patients admitted to medical or surgical ICUs with respiratory failure and/or shock (3). This cohort was restricted to a single center, Vanderbilt University Medical Center, for the present study. Patients who could not be assessed for delirium by study staff during the entirety of the hospital stay were excluded. Based on 513 patients analyzed, the GCS and RASS were strongly correlated (Spearman rho =0.806; 95% CI, 0.785–0.825) across all daily values. As expected, the neurologic component scores (SOFA-NeuroGCS and SOFA-NeuroRASS) were also correlated. The total SOFARASS scores were strongly correlated with the original (SOFAGCS) scores (Spearman rho =0.963; 95% CI, 0.956–0.968 for daily values) and at least moderately with other established illness severity scales.
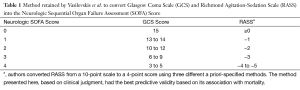
Full table
Both the mean SOFAGCS and the mean SOFARASS scores showed good discrimination for ICU mortality (AUC =0.799 and 0.814) and hospital mortality (AUC =0.771 and 0.782). They also showed a similar discrimination of these two outcomes when taking into account their maximum values. As noted by the authors, although the value for predicting mortality is statistically higher with the SOFARASS score, this difference probably remains clinically irrelevant.
This study highlights the difficulties in carrying out the GCS in ICU patients, often intubated (4), more or less sedated, and subject to indirect brain lesion as expressed by a varied symptomatology. Good examples of this difficulty are delirium, correlated with mortality (5), and hepatic encephalopathy (6). The search for efficient scores runs into the absence of a true gold standard that can be used to compare these scales. To our knowledge, there are no biological parameters or medical devices validated to compare these scales. The bispectral index (BIS) (7-11) has been shown to be able to predict the neurologic outcome in different settings in ICU patients. It could be used as a simple, reproducible and “physiologically based” method to assess the validity of these scales. This lack of a reference combines with a common methodological problem: the use of statistical correlations for comparing scales. Since these scores evaluate the same phenomena and are for the most part constructed in a similar manner, it makes sense that they evolve proportionally and are correlated. Their comparison should systematically integrate a comparative evaluation of their performance on clinical outcomes.
The RASS score is a rating scale for the quality of sedation and analgesia (2), similar to the RAMSAY score (12), but in two dimensions. These scores are dependent on the patient’s motor response capacity and are therefore corrupted by muscle relaxant drugs such as a curare. The BIS was developed to overcome this constraint. It has also been shown that the RASS and BIS were highly correlated (13). By analogy, they are used as a neurological severity score.
This new score, based on the RASS, is therefore a priori easier to administer in intubated (2) but non-curarized patients than the original and takes into account the agitation component.
The Vasilevskis et al. (1) paper provided a number of important contributions: first, there does not seem to be any loss of reliability in the short- and medium-term prediction of mortality for the patients in whom both scores are achievable. This result is reassuring in terms of its safety of use in routine practice and in future clinical studies. Second, the SOFARASS does not seem to score better than the classic SOFA for predictive capacity in these patients. It is likely that this score can be administered to a greater variety of patients, which was not assessed by the study. Most importantly, it can avoid the situation in which the SOFA score is underestimated because its neurological component is ignored.
The study has several limitations concerning the choice of the test population, which may limit the results in terms of the comparative performance of the two scores. It was restricted to patients with sepsis or respiratory distress, and sedation was not prospectively modified to allow assessment of the neurological component. While this choice is understandable for a first assessment, two points should be mentioned. First, the patients included were probably not those whose assessment of the neurological component is the most important for mortality (14). For example, patients with cardiac arrest causing anoxic brain injury were excluded, and in the BRAIN-ICU study (3), patients with neurologic disease or seizure as the initial diagnosis account for approximately 1% of the initial cohort. Second, the absence of sedative cessation for the evaluation of the motor component may cause an underestimation of neurological involvement evaluated by the RASS score. We can assume that an “agitated”, “very agitated” or “combative” patient as defined by the score has been administered greater sedation, which underestimates the difference with the GCS.
Notwithstanding the above limitations, Vasilevskis et al. have to be commended for their study. Their SOFARASS score looks promising for future studies in the assessment of symptom severity in critically ill patients, especially those with potential neurological damage (1). Further investigations should aim to assess whether the score can be used in a greater number of patients than the original score, and its predictive performance in a population of patients at high risk of neurological impairment, with a prospective design limiting the effect of sedation on that assessment.
Acknowledgements
None.
Footnote
Provenance: This is an invited article commissioned by the Section Editor Zhongheng Zhang (Department of Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, Jinhua, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vasilevskis EE, Pandharipande PP, Graves AJ, et al. Validity of a Modified Sequential Organ Failure Assessment Score Using the Richmond Agitation-Sedation Scale. Crit Care Med 2016;44:138-46. [Crossref] [PubMed]
- Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338-44. [Crossref] [PubMed]
- Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369:1306-16. [Crossref] [PubMed]
- Gorji MA, Gorji AM, Hosseini SH. Which score should be used in intubated patients' Glasgow coma scale or full outline of unresponsiveness? Int J Appl Basic Med Res 2015;5:92-5. [Crossref] [PubMed]
- Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291:1753-62. [Crossref] [PubMed]
- Orman ES, Perkins A, Ghabril M, et al. The confusion assessment method for the intensive care unit in patients with cirrhosis. Metab Brain Dis 2015;30:1063-71. [Crossref] [PubMed]
- Watson PL, Shintani AK, Tyson R, et al. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med 2008;36:3171-7. [Crossref] [PubMed]
- Dunham CM, Ransom KJ, McAuley CE, et al. Severe brain injury ICU outcomes are associated with Cranial-Arterial Pressure Index and noninvasive Bispectral Index and transcranial oxygen saturation: a prospective, preliminary study. Crit Care 2006;10:R159. [Crossref] [PubMed]
- Schnakers C, Ledoux D, Majerus S, et al. Diagnostic and prognostic use of bispectral index in coma, vegetative state and related disorders. Brain Inj 2008;22:926-31. [Crossref] [PubMed]
- Miao W, Zhang Y, Li H. Bispectral index predicts deaths within 2 weeks in coma patients, a better predictor than serum neuron-specific enolase or S100 protein. J Anesth 2013;27:855-61. [Crossref] [PubMed]
- Fàbregas N, Gambús PL, Valero R, et al. Can bispectral index monitoring predict recovery of consciousness in patients with severe brain injury? Anesthesiology 2004;101:43-51. [Crossref] [PubMed]
- Ramsay MA, Savege TM, Simpson BR, et al. Controlled sedation with alphaxalone-alphadolone. Br Med J 1974;2:656-9. [Crossref] [PubMed]
- Karamchandani K, Rewari V, Trikha A, et al. Bispectral index correlates well with Richmond agitation sedation scale in mechanically ventilated critically ill patients. J Anesth 2010;24:394-8. [Crossref] [PubMed]
- Yu A, Teitelbaum J, Scott J, et al. Evaluating pain, sedation, and delirium in the neurologically critically ill-feasibility and reliability of standardized tools: a multi-institutional study. Crit Care Med 2013;41:2002-7. [Crossref] [PubMed]


