Airway centered invasive pulmonary aspergillosis in an immunocompetent patient: case report and literature review
Introduction
Invasive pulmonary aspergillosis is caused by Aspergillus species, usually Aspergillus fumigatus, and involves the normal lung tissue. The diagnosis of proven IPA usually is based on histopathological confirmation with tissue invasion, including septated, acutely branching filamentous fungi, and a positive culture result for a specimen from a normally sterile site (1,2). It usually occurs in immunocompromised patients, especially in patients with neutropenia (2-4). Invasive pulmonary aspergillosis has been categorized into an angioinvasive and an airway centered invasive form depending on where Aspergillus mainly invades (2,4,5). Airway centered invasive aspergillosis accounts for 14−34% of the cases of invasive aspergillosis in immunocompromised patients (2,4,5). A few cases of angioinvasive pulmonary aspergillosis in individuals with normal immunity but without any chronic illness have been reported (6,7). However, very few cases of airway centered invasive aspergillosis in immunocompetent patients without chronic illness have been reported in the literature. Herein, we report the case of an immunocompetent patient with extensive airway centered invasive pulmonary aspergillosis.
Case presentation
A 29-year-old man visited a secondary hospital because of fever and dyspnea. He underwent chest radiography and computed tomography (CT). The physician suspected active tuberculosis or extensive bacterial pneumonia, and initiated treatment with anti-tuberculosis medication and empirical antibiotics. However, the patient was referred to our tertiary hospital because of persistent high fever and deterioration of his condition, as observed on his serial chest radiographs, despite the empirical treatment. The patient was healthy with no history of smoking, alcohol abuse, diabetes, or steroid or intravenous drug use. He denied any recent occupational or environmental exposure to dust. He was febrile with a body temperature of 38.2 °C. The results of his arterial blood-gas analysis were as follows: partial oxygen pressure (pO2) =73.4 mmHg and O2 saturation =96.1%. White blood cell count was elevated at 30.3×109/L with a differential neutrophil count of 93%, and his C-reactive protein level was elevated to 28.81 mg/dL (normal level: <0.30 mg/dL). The results of his renal and liver function tests were normal, except that his alkaline phosphatase level was elevated at 608 U/L (normal level: <335 U/L) and his albumin level was lowered at 2.3 g/dL (normal range: 3.1−5.2 g/dL).
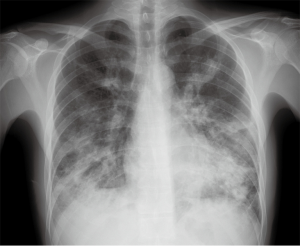
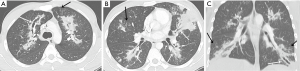
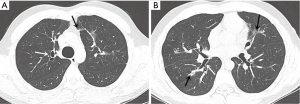
The initial chest radiograph obtained at our hospital showed extensive peribronchial consolidation in both the lungs, with lower lobe dominance (Figure 1). A subsequent CT scan showed extensive peribronchial consolidation with dilated subsegmental bronchi, accompanied by poorly defined centrilobular nodules and branching linear nodules, resembling a “tree-in-bud,” near the peribronchial lesion in both the lungs (Figure 2). The peribronchial lesion was unresponsive to the empirical treatment that was administered for 6 days and had rather rapidly aggravated from the time of the initial CT scan, which was performed at the previous hospital. Because the CT scan showed extensive peribronchial consolidation with severe destruction of the bronchial wall, which are not typical imaging features of pulmonary tuberculosis, we suspected airway centered invasive pulmonary aspergillosis and performed bronchoscopy, although the patient was healthy without any underlying disease.
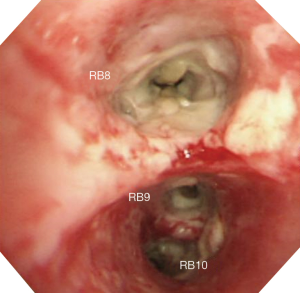
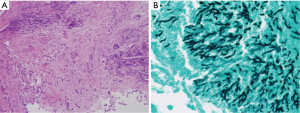
Bronchoscopy revealed that the bronchial wall was covered by purulent exudates (Figure 3). A bronchoscopic biopsy specimen was obtained from the right lower lobar bronchus, and bronchial secretion was collected for sputum culture. Histopathological examination of the bronchoscopic biopsy specimen showed extensive tissue necrosis associated with infiltration of fungal hyphae. Staining with the periodic acid–Schiff stain or Grocott’s methenamine silver showed regular, narrow branching, and septate hyphae of Aspergillus (Figure 4). The results of Ziehl-Neelsen staining of acid-fast bacilli were negative. The culture of the bronchial washing was positive for Aspergillus fumigatus.
The results of the test for the human immunodeficiency virus (HIV) were negative. The patient did not have a history of chronic recurrent infection. The patient’s immunoglobulin level was within the normal limit. An antifungal drug regimen of intravenous voriconazole (4 mg/kg q 12 h after 6 mg/kg q 12 h for 2 days) was initiated. The patient’s condition substantially improved. Two months later, a follow-up CT scan was obtained. Marked disappearance of the peribronchial consolidation and poorly defined centrilobular nodules were observed, but multiple bronchiectasis with some fibrosis and parenchymal distortion were observed in both the lungs (Figure 5). Two days later, the patient was discharged, and his treatment was switched to oral voriconazole (400 mg per day).
Discussion
Invasive pulmonary aspergillosis, including the angioinvasive and airway centered invasive type, typically occurs in exclusively immunocompromised patients, especially those with neutropenia and those with HIV infection, after solid organ transplantation, who receive immunosuppressive or steroid therapy for chronic granulomatous disease (2,4,7,8). Whereas recently, the incidence of invasive pulmonary aspergillosis in immunocompetent patients with critical illness, such as those with postinfectious condition, or in immunocompetent patients with occupational exposure to dust, such as those who work at construction sites, has increased (9,10). Recently, invasive aspergillosis is on the rise and very difficult to diagnose in patients with severe critical illness (but without chronic underlying disease and certainly without host factors as defined by the EORTC/Mycosis Study Group). Especially when the patient is mechanically ventilated, medical imaging is particularly problematic due to non-specific imaging finding in most of those patients (11,12). However, the incidence among immunocompetent patients without acute or chronic critical illness is rather low (9,10). In immunocompetent patients even without chronic underlying disease such as diabetes mellitus, airway centered invasive pulmonary aspergillosis occurs very rarely, and, to our knowledge, a few such cases have been reported in the literature (13-15).
Clinical symptoms in patients with invasive pulmonary aspergillosis include fever, cough, dyspnea, hemoptysis, and pleuritic chest pain However, these clinical symptoms without the classical risk factor are nonspecific; thus, accurate diagnosis could be often delayed. The culture of Aspergillus from a site that is normally sterile in tandem with histological evidence of tissue invasion by fungal hyphae is regarded as the “gold standard” (9). However, acquisition of tissue specimens from critically ill patients without clinical suspicion is not always possible. Whereas noninvasive tests for a serologic marker such as galactomannan testing or Aspergillus polymerase chain reaction assay are other good options for the diagnosis of invasive aspergillosis (9), these tests cannot be performed clinically in the patients without a history of immune suppression or an underlying disease.
Kousha et al. reported that the use of CT scan in the course of invasive pulmonary aspergillosis leads to earlier diagnosis and improved outcomes (16). Furthermore, depending on whether Aspergillus chiefly invades a vessel or the airway, radiological patterns on CT scan were classified as angioinvasive or airway centered invasive, respectively (4,5). According to previous studies, CT scan showed patchy bilateral consolidation, predominantly peribronchial in location; centrilobular nodules; and, in some cases, a “tree-in-bud” appearance (2,5). Park et al. suggested that the typical diagnostic findings of the airway centered invasive form are patchy peribronchial consolidation, small airway lesions including centrilobular nodules, and bronchiectasis (17). The main CT findings in our case were extensive bilateral patchy consolidation with irregularly dilated segmental or subsegmental bronchi, and centrilobular nodules with a mainly branching pattern, consistent with the CT findings of extensive airway centered invasive aspergillosis. The radiologic findings and the severity of the disease in our patient were consistent with the previous findings in immunocompetent young individuals with airway centered invasive pulmonary aspergillosis (13). Because cavity lesions and centrilobular nodules with a branching pattern were also observed on the CT scan, and the patient did not have a history of an immunosuppressive condition, the physician in the previous hospital misdiagnosed the patient’s condition as active tuberculosis, similar to a previously reported case (13). However, we suggest that extensive peribronchial consolidation with dilated segmental or subsegmental bronchi is not a common finding in active tuberculosis.
Although the exact mechanism by which the Aspergillus species causes invasive aspergillosis in immunocompetent patients is not understood, the species may inhibit antigen-presenting cell functions and suppress T cell response by a toxic substance, gliotoxin which Aspergillus fumigatus produces (18).
At present, voriconazole is considered the therapy of choice with better tolerance to side effects compared with those including nephrotoxicity, hepatotoxicity, and acute infusion-related toxic effects of classical antifungal drug, amphotericin B, and therapy with echinocandins, such as caspofungin, is used as a salvage therapy for patients who show intolerance to the first-line therapy (9). With only voriconazole treatment for 2 months, the patient’s condition improved substantially, and the pulmonary lesion alleviated remarkably with a little postinflammatory sequelae, including bronchiectasis and fibrosis, and minimal parenchymal distortion despite the extensive pulmonary involvement, as observed on the initial CT scan.
We experienced a rare case of an immunocompetent patient with airway centered invasive pulmonary aspergillosis that was suspected on the basis of the CT findings and was confirmed on the basis of the bronchoscopic biopsy examination. We proposed that if the characteristic imaging findings such as patchy peribronchial consolidation, bronchiectasis, small airway lesions including centrilobular nodules are observed on a CT scan, airway centered invasive pulmonary aspergillosis should be included in the differential diagnosis even in the case of immunocompetent patients.
Acknowledgements
Funding: This work was supported by INHA University research fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. [Crossref] [PubMed]
- Franquet T, Müller NL, Oikonomou A, et al. Aspergillus infection of the airways: computed tomography and pathologic findings. J Comput Assist Tomogr 2004;28:10-6. [Crossref] [PubMed]
- Tunnicliffe G, Schomberg L, Walsh S, et al. Airway and parenchymal manifestations of pulmonary aspergillosis. Respir Med 2013;107:1113-23. [Crossref] [PubMed]
- Krenke R, Grabczak EM. Tracheobronchial manifestations of Aspergillus infections. ScientificWorldJournal 2011;11:2310-29. [Crossref] [PubMed]
- Logan PM, Primack SL, Miller RR, et al. Invasive aspergillosis of the airways: radiographic, CT, and pathologic findings. Radiology 1994;193:383-8. [Crossref] [PubMed]
- Yoon SH, Park CM, Goo JM, et al. Pulmonary aspergillosis in immunocompetent patients without air-meniscus sign and underlying lung disease: CT findings and histopathologic features. Acta Radiol 2011;52:756-61. [Crossref] [PubMed]
- Ko JP, Kim DH, Shepard JA. Pulmonary aspergillosis in an immunocompetent patient. J Thorac Imaging 2002;17:70-3. [Crossref] [PubMed]
- Chotirmall SH, Al-Alawi M, Mirkovic B, et al. Aspergillus-associated airway disease, inflammation, and the innate immune response. Biomed Res Int 2013;2013:723129.
- Reichenberger F, Habicht JM, Gratwohl A, et al. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J 2002;19:743-55. [Crossref] [PubMed]
- Park DW, Yhi JY, Koo G, et al. Fatal clinical course of probable invasive pulmonary aspergillosis with influenza B infection in an immunocompetent patient. Tuberc Respir Dis (Seoul) 2014;77:141-4. [Crossref] [PubMed]
- Taccone FS, Van den Abeele AM, Bulpa P, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care 2015;19:7. [Crossref] [PubMed]
- Vandewoude K, Blot S, Benoit D, et al. Invasive aspergillosis in critically ill patients: analysis of risk factors for acquisition and mortality. Acta Clin Belg 2004;59:251-7. [Crossref] [PubMed]
- Xu XY, Sun HM, Zhao BL, et al. Diagnosis of airway-invasive pulmonary aspergillosis by tree-in-bud sign in an immunocompetent patient: case report and literature review. J Mycol Med 2013;23:64-9. [Crossref] [PubMed]
- Mohan A, Guleria R, Mukhopadhyaya S, et al. Invasive tracheobronchial aspergillosis in an immunocompetent person. Am J Med Sci 2005;329:107-9. [Crossref] [PubMed]
- De S, Desikan P. Unusual cause of chronic cough in an immunocompetent host. J Bronchology Interv Pulmonol 2009;16:61-2. [Crossref] [PubMed]
- Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev 2011;20:156-74. [Crossref] [PubMed]
- Park SY, Lim C, Lee SO, et al. Computed tomography findings in invasive pulmonary aspergillosis in non-neutropenic transplant recipients and neutropenic patients, and their prognostic value. J Infect 2011;63:447-56. [Crossref] [PubMed]
- Stanzani M, Orciuolo E, Lewis R, et al. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 2005;105:2258-65. [Crossref] [PubMed]


