Bioprosthetic versus mechanical prostheses for valve replacement in end-stage renal disease patients: systematic review and meta-analysis
Introduction
The prevalence of heart valve disease is increasing in patients with ESRD requiring dialysis (1,2). There has also been a corresponding increase in the number of dialysis patients presenting for cardiac valve surgery (1,3,4). However, such a patient population presents a challenging dilemma for the clinician and surgeon on deciding which prosthesis type is most suitable. The American College of Cardiology and American Heart Association society guidelines (5) have previously recommended the use of mechanical prostheses in end-stage renal disease (ESRD) patients requiring dialysis, quoting accelerated valve calcification and structural deterioration as caveats of bioprosthesis. However, these recommendations have been controversial given that recent long-term studies have demonstrated no major differences in survival outcomes and valve durability in bioprosthesis versus mechanical valves end-stage renal failure patients. This may have significant clinical implications for dialysis patients requiring valve replacements, given their poor long-term survival. Indeed, current guidelines have now encouraged individual patient-based valve selection (6). In comparison to bioprosthesis, mechanical prostheses are associated with lifelong anticoagulation therapy as well as potential increased risk of strokes and thromboembolism.
The present literature is currently limited to smaller retrospective and prospective studies, with mixed aortic and mitral valve replacement cohorts. In a recent analysis of 406 patients over a 15 years interval, Okada and colleagues (7) demonstrated no difference in late survival or bleeding complications between biological and mechanical prosthesis groups. Even in dialysis patients, when the prostheses type was selected according to the guidelines for non-dialysis patients, no differences were observed in valve-related complications between biological and mechanical prosthesis groups. Another analysis of 202 patients demonstrated comparable long-term survival up to 10 years between bioprosthetic versus mechanical valve replacement. In order to assess the relative benefits and risks of bioprosthetic versus mechanical prostheses in ESRD patients requiring dialysis, a systematic review and meta-analysis of the literature was performed.
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for the present systematic review (8,9). Electronic searches were performed using Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club, and Database of Abstracts of Review of Effectiveness (DARE), from their dates of inception to February 2015. To achieve maximum sensitivity of the search strategy, we combined the terms “chronic renal failure”, “dialysis”, “ESRD”, “valve-replacement”, “heart”, or “prosthesis” which were searched as text words and exploded as MeSH headings where possible (Table S1). Two authors performed the search independently, and any discrepancies were resolved by discussion. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies, assessed using the inclusion and exclusion criteria.

Full table
Selection criteria
Eligible studies for the present systematic review and meta-analysis were those which compared bioprosthesis with mechanical prostheses in patients with ESRD receiving dialysis. Studies that did not include mortality or complications as endpoints were excluded. Studies with fewer than 10 patients in each cohort were also excluded. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment. All publications were limited to those involving human subjects and in the English language. Abstracts, case reports, conference presentations, editorials, reviews and expert opinions were excluded.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. Two investigators independently reviewed each retrieved article. Discrepancies between the two reviewers were resolved by discussion and consensus. The final results were reviewed by the senior investigators.
Statistical analysis
Clinical outcomes were assessed using standard meta-analysis techniques, with the hazard ratio (HR) used as a summary statistic to compare actuarial survival between prosthesis groups. Analysis was performed by estimating HR and 95% confidence interval (CI) for longer-term survival outcomes from available survival curves and tables by using a spreadsheet developed by Tierney and collaborators. Pooled estimates of mean effect of prosthesis type on long-term survival (pooled HR) in patients with ESRD and the corresponding 95% CI were determined by using inverse variance fixed-effects model and the DerSimonian and Laird random-effects models.
Relative risk (RR) was used as a summary statistic for dichotomous variables, and weighted mean difference (WMD) was used for continuous variables. In the present study, both fixed-effect and random-effects models were tested. In the fixed-effects model, it was assumed that treatment effect in each study was the same, whereas in a random-effects model, it was assumed that there were variations between studies. χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance, with values greater than 50% considered as substantial heterogeneity. In the present meta-analysis the results using the random-effects model were presented to take into account the possible clinical diversity and methodologic variation between studies. All P values were 2-sided. All statistical analysis was conducted with Review Manager Version 5.2.2 (Cochrane Collaboration, Software Update, Oxford, UK).
Publication bias
Evidence of publication bias was sought using Begg and Egger methods. Possible asymmetry was investigated using trim-and-fill analysis.
Results
Literature search
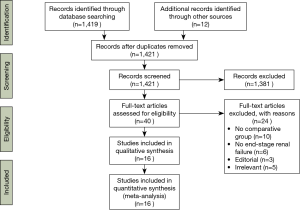
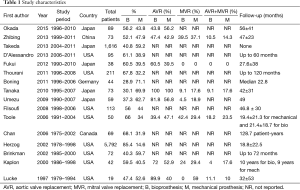
A total of 1,421 studies were identified through six electronic database searches and from other sources including reference lists (Figure 1). After exclusion of duplicate or irrelevant references, 40 potentially relevant articles were retrieved. After application of inclusion and exclusion criteria, 16 relevant articles were included in the present systematic review and meta-analysis (7,10-24). A total of 8,483 patients with ESRD were included for analysis, including 6,187 receiving bioprosthesis and 2,296 receiving mechanical valves. Study characteristics are summarized in Table 1.
Full table
Patient characteristics
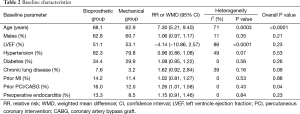
Baseline characteristics of included patients are summarized in Table 2. Similar baseline characteristics were observed in both comparison arms. Males accounted for an average of 62.8% in the bioprosthetic group and 60.7% in the mechanical group (P=0.21). The mean of the average age in each study ranged was 68.1 in the bioprosthetic group and 62.9 in the mechanical group (68.1% vs. 62.9%, P<0.0001). There was no significant difference between the bioprosthetic and mechanical groups in the proportion of patients with left ventricle ejection fraction (LVEF) (51.1% vs. 53.1%, P=0.23), hypertension (82.3% vs. 79.8%, P=0.53), diabetes (34.4% vs. 29.9%, P=0.26), chronic lung disease (7.6% vs. 3.2%, P=0.09), prior myocardial infection (MI) (14.2% vs. 11.4%, P=0.88) and perioperative endocarditis (13.3% vs. 8.5%, P=0.23). Prior angioplasty by percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery was significantly higher in the bioprosthetic group compared to the mechanical group (16.0% vs. 12.0%, P=0.04).
Full table
Short and long-term survival
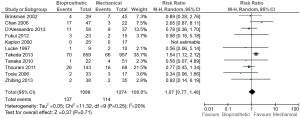
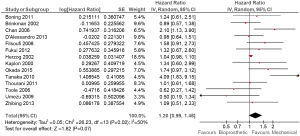
The risk of 30-day all-cause mortality was not significantly different between the bioprosthetic and mechanical groups (12.6% vs. 8.9%, RR, 1.07; 95% CI, 0.77–1.48; P=0.71; I2=20%; Figure 2). Furthermore, all-cause mortality was also not significantly different (HR, 1.20; 95% CI, 0.99–1.46; P=0.07; I2=50%; Figure 3). No significant heterogeneity was observed in these two comparisons.
Complications
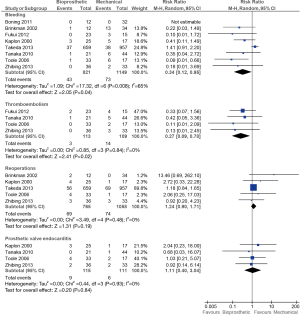
Bleeding complications were reported in eight out of sixteen included studies. The frequency of bleeding was significantly lower in the bioprosthetic group compared to the mechanical group (5.2% vs. 6.4%; RR, 0.34; 95% CI, 0.12–0.95; P=0.04; I2=65%). Four studies reported outcomes for thromboembolism. Overall, thromboembolism was significantly lower in the bioprosthetic group compared to the mechanical group (2.6% vs. 12.8%; RR, 0.27; 95% CI, 0.09–0.78; P=0.02; I2=0%). There was no significant difference in the proportion of patients with reoperations between the bioprosthetic and the mechanical group (9.1% vs. 7.0%; RR, 1.24; 95% CI, 0.90–1.71; P=0.19; I2=0%). Additionally, the incidence of prosthetic valve endocarditis was similar between both groups (7.8% vs. 5.4%; RR, 1.11; 95% CI, 0.40–3.04; P=0.84; I2=0%) (Figure 4).
Publication bias
Funnel plot analysis of mid-long term survival outcomes did not demonstrate significant asymmetry, by either Begg’s methods (P=0.58, two-sided) or Egger’s methods (P=0.21, two-sided). Trim-and-fill analysis using random-effects models did not demonstrate any “missing studies” in the analysis. Thus, publication bias was not an influencing factor on mid-long term survival analysis.
Discussion
With advancing medical therapy, patients receiving dialysis are expected to live with a longer life expectancy and present with multiple comorbidities. Due to the ageing population, an increasing number of patients with chronic kidney disease will require valve replacement surgery (4,24). The presence and progression of cardiovascular and renal disease are related because of multiple shared risk factors (25). In this population, degenerative valvular disease may occur earlier than in patients with normal renal function. Therefore, careful risk assessment and choice of valve prosthesis should be conducted prior to surgical intervention (26).
We performed this updated meta-analysis with 8,483 patients with ESRD receiving dialysis requiring valve replacement operations to compare various outcomes between bioprosthesis and mechanical valves. The results of the present meta-analysis suggest no difference in 30-day mortality and mid-term survival between patients receiving bioprosthesis and mechanical valves. The implantation of bioprosthesis may be appropriate for patients with ESRD undergoing valve replacement surgery.
This meta-analysis showed no significant difference in 30-day mortality between bioprosthesis and mechanical valves (12.6% vs. 8.9%). Of the 11 studies analysed, one study (11) demonstrated decreased mortality in favour of mechanical prostheses over bioprosthesis and the remaining 10 studies failed to demonstrate a difference. There was no significant difference in mid-term survival between bioprosthesis and mechanical valves. Of the 14 included studies, two studies (16,20) demonstrated a survival advantage in favour of mechanical prostheses over bioprosthesis and the remaining 12 studies failed to demonstrate a survival advantage according to prosthesis type. There was a significant reduction in bleeding events comparing bioprosthesis with mechanical valves (5.2% vs. 6.4%). The results also confirmed a significant reduction in thromboembolism with bioprosthesis as compared to mechanical valves (2.7% vs. 12.8%).
In terms of valve selection for surgery, there are a number of advantages and disadvantages associated with both bioprosthetic and mechanical valves. The main advantage with bioprosthetic valves is that patients do not require life-long anti-coagulation therapy due to the lowered thrombotic risk compared to mechanical valves (27,28). As such, patients with bioprosthetic valves have a significantly decreased risk of bleeding (27). Despite its decreased bleeding risk, opponents have quoted that bioprosthetic valves are less durable than mechanical valves, which remains a major disadvantage (29). Due to structural deterioration of the bioprosthetic valve, there is an increased risk of reoperation for these patients. In a population aged 60 and above, the likelihood of reoperation is reduced since the lifespan of tissue valves is likely to exceed the expected life expectancy of patients (29). In comparison, mechanical valves have greater durability (20–30 years) than bioprosthetic valves (10–15 years). The main disadvantage with mechanical valves is that patients need to be on life-long warfarin and are at an increased risk of bleeding and thromboembolism (30).
For the above reasons, there has been much debate over the choice of prostheses in patients on dialysis undergoing cardiac valve replacement surgery. A number of factors must be considered when choosing between a bioprosthetic and a mechanical valve (7) for dialysis patients, particularly the expected survival rate, risk of bleeding complications, and the potential for accelerated structural valve deterioration. In our meta-analysis, patients with bioprosthetic valves did not require long-term anticoagulation and experienced fewer bleeding events and lower rates of thromboembolism. The recent development of valve-in-valve transcatheter aortic valve implantation is a viable future option for patients with failed bioprosthetic valves.
In patients with chronic renal disease, the progression of aortic stenosis is accelerated compared with patients with normal renal function due to derangements in calcium and phosphate metabolism (21). Early concerns were raised about the potential increased rate of valve calcification in ESRD patients. The use of bioprosthetic valves was considered harmful in ESRD patients for decades (26). More recently, it has been suggested that there is no convincing evidence for accelerated calcification as a major cause of bioprosthetic valve failure and resultant adverse morbidity and mortality (23). A number of recent studies have demonstrated equivalent survival rates between bioprosthetic and mechanical valves dialysis patients (20). Our meta-analysis also confirms with previous studies by demonstrating the same equivalence of mortality and survival rates among valve prosthesis choice (31).
Despite early concerns about rapid calcification of bioprosthetic valves in dialysis patients, guidelines have been changed following multiple studies demonstrating equivalent outcomes between valve types. In the 2006 Guidelines, the description of valve selection for dialysis patients was removed from the 1998 American College of Cardiology/American Heart Association Task Force Guidelines, where the use of bioprosthesis for dialysis patients was classified as a type III indication (5). Guidelines from the National Kidney Foundation’s National Disease Outcomes Quality Initiative no longer discourage the implantation of bioprosthetic valves in patients with end-stage renal failure.
The present meta-analysis is constrained by several limitations. Firstly, the present meta-analysis included several retrospective studies with small sample sizes. The lack of blinding and randomization may increase risk of selection bias. The relatively small sample size reduces statistical power, and thus differences in some complication outcomes may not be detected. There were also differences in the underlying baseline characteristics of the two cohorts, with the bioprosthetic group being older by 5 years and having a higher proportion with prior revascularization procedures, which may have an impact on the reported outcomes. Furthermore, unadjusted summary estimates were used for meta-analysis. As such, the effect of potential confounders on outcomes cannot be ruled out. Given the lack of stratification between aortic and mitral prosthesis in the included studies, subgroup analysis to elucidate any differences between these two valve positions in terms of outcomes could not be performed.
Conclusions
In summary, mid-long term survival of patients with ESRD is not dependent on the type of prosthesis, whether bioprosthetic or mechanical. In terms of complications, there appears to be lower rates of bleeding and thromboembolic rates in the bioprosthetic group, but similar reoperations and valve endocarditis between the two cohorts. Whilst further studies are required to differentiate the impact of valve location, the presented results are likely to be applicable for ESRD patients requiring aortic valve replacement.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kramer A, Stel V, Zoccali C, et al. An update on renal replacement therapy in Europe: ERA-EDTA Registry data from 1997 to 2006. Nephrol Dial Transplant 2009;24:3557-66. [Crossref] [PubMed]
- Barrett BJ, Parfrey PS, Morgan J, et al. Prediction of early death in end-stage renal disease patients starting dialysis. Am J Kidney Dis 1997;29:214-22. [Crossref] [PubMed]
- Takami Y, Tajima K, Okada N, et al. Simplified management of hemodialysis-dependent patients undergoing cardiac surgery. Ann Thorac Surg 2009;88:1515-9. [Crossref] [PubMed]
- Penta de Peppo A, Nardi P, De Paulis R, et al. Cardiac surgery in moderate to end-stage renal failure: analysis of risk factors. Ann Thorac Surg 2002;74:378-83. [Crossref] [PubMed]
- Bonow RO, Carabello B, de Leon AC Jr, et al. Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation 1998;98:1949-84. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521-643. [Crossref] [PubMed]
- Okada N, Tajima K, Takami Y, et al. Valve selection for the aortic position in dialysis patients. Ann Thorac Surg 2015;99:1524-31. [Crossref] [PubMed]
- Phan K, Tian DH, Cao C, et al. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg 2015;4:112-22. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Zhibing Q, Xin C, Ming X, et al. Should bioprostheses be considered the valve of choice for dialysis-dependent patients? J Cardiothorac Surg 2013;8:42. [Crossref] [PubMed]
- Takeda K, Miyata H, Motomura N, et al. Contemporary perioperative results of heart valve replacement in dialysis patients: analysis of 1,616 patients from the Japan adult cardiovascular surgery database. J Heart Valve Dis 2013;22:850-8. [PubMed]
- D'Alessandro DA, Skripochnik E, Neragi-Miandoab S. The significance of prosthesis type on survival following valve replacement in dialysis patients. J Heart Valve Dis 2013;22:743-50. [PubMed]
- Fukui S, Yamamura M, Mitsuno M, et al. Aortic valve prosthesis selection in dialysis patients based on the patient's condition. J Artif Organs 2012;15:162-7. [Crossref] [PubMed]
- Thourani VH, Sarin EL, Keeling WB, et al. Long-term survival for patients with preoperative renal failure undergoing bioprosthetic or mechanical valve replacement. Ann Thorac Surg 2011;91:1127-34. [Crossref] [PubMed]
- Böning A, Boedeker RH, Rosendahl UP, et al. Long-term results of mechanical and biological heart valves in dialysis and non-dialysis patients. Thorac Cardiovasc Surg 2011;59:454-9. [Crossref] [PubMed]
- Tanaka K, Tajima K, Takami Y, et al. Early and late outcomes of aortic valve replacement in dialysis patients. Ann Thorac Surg 2010;89:65-70. [Crossref] [PubMed]
- Umezu K, Saito S, Yamazaki K, et al. Cardiac valvular surgery in dialysis patients: comparison of surgical outcome for mechanical versus bioprosthetic valves. Gen Thorac Cardiovasc Surg 2009;57:197-202. [Crossref] [PubMed]
- Filsoufi F, Chikwe J, Castillo JG, et al. Prosthesis type has minimal impact on survival after valve surgery in patients with moderate to end-stage renal failure. Nephrol Dial Transplant 2008;23:3613-21. [Crossref] [PubMed]
- Toole JM, Stroud MR, Kratz JM, et al. Valve surgery in renal dialysis patients. J Heart Valve Dis 2006;15:453-8; discussion 458. [PubMed]
- Chan V, Jamieson WR, Fleisher AG, et al. Valve replacement surgery in end-stage renal failure: mechanical prostheses versus bioprostheses. Ann Thorac Surg 2006;81:857-62. [Crossref] [PubMed]
- Herzog CA, Ma JZ, Collins AJ. Long-term survival of dialysis patients in the United States with prosthetic heart valves: should ACC/AHA practice guidelines on valve selection be modified? Circulation 2002;105:1336-41. [Crossref] [PubMed]
- Brinkman WT, Williams WH, Guyton RA, et al. Valve replacement in patients on chronic renal dialysis: implications for valve prosthesis selection. Ann Thorac Surg 2002;74:37-42; discussion 42. [Crossref] [PubMed]
- Kaplon RJ, Cosgrove DM 3rd, Gillinov AM, et al. Cardiac valve replacement in patients on dialysis: influence of prosthesis on survival. Ann Thorac Surg 2000;70:438-41. [Crossref] [PubMed]
- Lucke JC, Samy RN, Atkins BZ, et al. Results of valve replacement with mechanical and biological prostheses in chronic renal dialysis patients. Ann Thorac Surg 1997;64:129-32; discussion 132-3. [Crossref] [PubMed]
- Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci 2003;325:163-7. [Crossref] [PubMed]
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e1-142. [Crossref] [PubMed]
- van Geldorp MW, Eric Jamieson WR, Kappetein AP, et al. Patient outcome after aortic valve replacement with a mechanical or biological prosthesis: weighing lifetime anticoagulant-related event risk against reoperation risk. J Thorac Cardiovasc Surg 2009;137:881-6, 886e1-5.
- Rahimtoola SH. Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol 2003;41:893-904. [Crossref] [PubMed]
- Puvimanasinghe JP, Steyerberg EW, Takkenberg JJ, et al. Prognosis after aortic valve replacement with a bioprosthesis: predictions based on meta-analysis and microsimulation. Circulation 2001;103:1535-41. [Crossref] [PubMed]
- Tillquist MN, Maddox TM. Cardiac crossroads: deciding between mechanical or bioprosthetic heart valve replacement. Patient Prefer Adherence 2011;5:91-9. [Crossref] [PubMed]
- Chan V, Chen L, Mesana L, et al. Heart valve prosthesis selection in patients with end-stage renal disease requiring dialysis: a systematic review and meta-analysis. Heart 2011;97:2033-7. [Crossref] [PubMed]