The equivalent efficacy of multiple operations for multiple primary lung cancer and a single operation for single primary lung cancer
Introduction
Multiple primary lung cancer (MPLC) is a special type of non-small cell lung cancer. Although the differential diagnosis of MPLC from stage IV lung cancer remains controversial, MPLC can diagnosed according to the Martini-Melamed criteria in most cases (1). According to the TNM Classification system (7th edition, 2009), the lesions of MPLC should be staged independently and the higher-stage lesion should be regarded as the major lesion (2,3). The appropriate treatment should be determined accordingly,most researchers recommend surgery as the choice of treatment when conditions permit. Nevertheless, only few studies have reported on the long-term survival of patients with MPLC after surgical treatment. We hypothesized that the efficacy of surgical treatment for early-stage MPLC is equivalent to that of early-stage single primary lung cancer. In this study, we report on the surgical treatment of 31 patients with MPLC who were among 1,290 patients with non-small cell lung cancer who underwent surgery performed by a single surgeon group. Our report particularly focuses on the long-term survival of these 31 patients.
Methods
Study subjects
This study was approved by both the Ethics and the Academic committees of Peking University School of Oncology, and informed consent was obtained from all patients. All patient data were retrieved from our prospective database of patients with lung cancer treated by a single surgeon group at Beijing Cancer Hospital, Peking University. Between January 2000 and July 2013, 1,290 patients with lung cancer underwent surgery. Of these, 31 patients were diagnosed with MPLC according to the Martini-Melamed criteria (1).
The Martini-Melamed criteria were as follows: (I) tumors located in different segments, lobes, or lungs; (II) tumors developed independently from carcinoma in situ; (III) no evidence of tumor cells at the common lymphatic drainage site and no extrapulmonary metastasis observed at diagnosis; and (IV) synchronous or metachronous lung cancers, with similar or different histology and unique pathological characteristics. The Union for International Cancer Control TNM Classification of Malignant Tumors (7th Edition, 2009) was used for the pathological staging of each lesion. The exclusion criteria were as follows: (I) the presence of extrapulmonary malignancy or (II) a clear previous history of malignancy (possibility of the lung lesions being metastatic).
In this study, 31 patients diagnosed with MPLC accounted for 2.4% (31/1,290). Among them, 13 were male (41.9%) and 18 were female (58.1%). The median age was 60 years (range, 35–75 years). Twenty patients (38.7%) were smokers. Twenty-seven patients (87.1%) had synchronous tumors, and 4 (12.9%) had metachronous tumors. 15 patients (48.4%) had contralateral tumors, and 16 patients (51.6%) had ipsilateral tumors in different lobes.
Follow-up examinations
Follow-up examinations were conducted every 3 months for the first 2 years, every 6 months from 2 to 5 years, and once a year after 5 years postoperatively. The follow-up examination for metachronous MPLCs began 3 months again, after the second surgery. Preoperative and postoperative examinations included thoracic enhanced CT, head enhanced MRI/CT, bronchoscopy, bone scintigraphy, ultrasonography analysis of the supraclavicular region and abdomen, and whole body PET-CT/CT. Some patients underwent transbronchial needle aspiration, endobronchial ultrasonography, or mediastinoscopy. For these 31 patients, the follow-up duration was calculated from the first surgery for synchronous tumors and from the secondary tumor surgery for metachronous tumors. In this database, the last follow-up date was June 30, 2015, or the date of death, whichever came first. Follow up was completed in all patients. The median follow-up duration was 34.0 months (range, 17.7–167.6 months).
Statistical analysis
Statistical analysis was performed using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). Survival curves were analyzed using the Kaplan-Meier method and compared using the log-rank test. A P value of <0.05 was considered statistically significant.
Results
Surgical strategy
The operation sequence varied depending on whether the tumor was synchronous or metachronous, ipsilateral or contralateral, and major or minor, and on the pulmonary function of the major and minor lobes (Table 1).
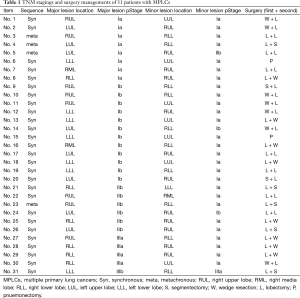
Full table
- Two patients (6.5%) had independent synchronous tumors in the left upper and left lower lobes of the lung. In both cases, sublobectomy was not feasible for any lesion; therefore, left pneumonectomy was performed.
- Ten patients (32.3%) had synchronous ipsilateral tumors in different lobes; however, the major lesion could be distinguished from the minor lesion. These patients underwent sublobectomy to remove the minor lesions and lobectomy to remove the major lesions. Two patients (6.5%) underwent bilobectomy because sublobectomy could not be applied to remove any right-sided lesions; this comprised upper and middle bilobectomy in one case and middle and lower bilobectomy in the other case.
- For the 10 patients (32.3%) who had synchronous contralateral lesions, where the major and minor lesions could be distinguished, sublobectomy was performed for the minor lesions, followed by lobectomy for the major lesions in the contralateral lobes.
- For the 3 patients (9.7%) who had synchronous contralateral lesions, in whom sublobectomy could not be performed, lobectomy was performed on both sides in a stepwise manner.
- Of the 4 patients (12.9%) who had metachronous MPLC, 2 (6.5%) had ipsilateral lesions in different lobes, which occurred successively in the right upper and right lower lobes. One patient underwent lobectomy for the primary lesion and sublobectomy for the secondary lesions; the other patient underwent lobectomy on the residual lobe. Two patients (6.5%) had contralateral lesions. For 1 patient, the primary lesion was removed via lobectomy, and the secondary lesion was removed via sublobectomy. For the other patient, lobectomy was performed.
Histopathological analysis and TNM stages of the 31 patients
Pathological diagnosis was performed in accordance with the fourth revision of the World Health Organization Classification of Lung Tumors [2004], and lung adenocarcinoma was diagnosed according to the new international multidisciplinary classification of lung adenocarcinoma (4-6). Both the major and minor lesions in each of all 31 patients showed same pathological type by hematoxylin-eosin staining. Of the 31 cases, 24 (77.4%) were lung adenocarcinoma and 7 (22.6%) were other tumor types. Postoperative pathological staging was performed for all patients according to the TNM Classification of Lung cancer (7th edition, 2009), and multiple primary lesions were staged separately (Table 1).
Survival analysis
The database included 1,290 cases of lung cancer, for which the 1-, 3- and 5-year were 95.4%, 80.5%, and 66.2%, respectively. The analysis based on TNM staging showed that the 5-year of the patients with stage I, II, and III cancers were 80.8%, 66.1%, and 45.0%, respectively. The 1-, 3- and 5-year postoperative survival rates for the 31 patients with MPLC were 100%, 75.8% and 75.8%, respectively (Figure 1). According to the TNM classification, patients with stage I cancer (20 cases) had 1-, 3- and 5-year of 100%, 77.2% and 77.2%, respectively (Figure 2). Patients with different pathological stages at risk were listed in Table 2. There were no statistically significant in survivance between MPLC and single primary lung cancer (P=0.455).
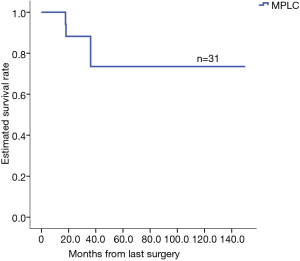
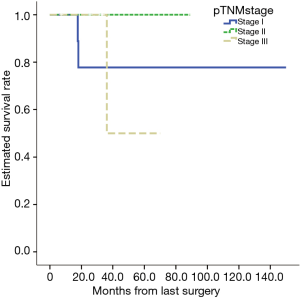

Full table
Discussion
Diagnosis of the multiple primary lung cancer (MPLC) stage
As early as in 1924, Beyreuther described the first case of double primary lung cancer, diagnosed on the basis of autopsy findings. In 1935, Lindburg et al. (4) reported the first clinical diagnosis of double primary lung cancer. In 1967, Auerbach et al. (6) reported that the incidence of MPLC was between 3.5% and 14.5%, on the basis of autopsy results. Currently, MPLC accounts for approximately 0.2–20% of reported lung cancer cases (7) and for 2.4% of the entire cohort (31/1,290) in this study. In 1975, Martini and Melamed (1) first developed diagnostic criteria for MPLC. In 2007, the American College of Chest Physicians developed new diagnostic criteria for MPLC (8), which provided clearer quantification and could be easily applied in clinical practice. However, lesions within the same lobe and multiple lesions with lymph node metastasis were both classified as intrapulmonary metastasis, resulting in an overly strict standard. Therefore, some patients may miss the opportunity for surgery. Although patients in this group were diagnosed according to the Martini-Melamed criteria, five patients with stage IIIA (N2) lesions were also considered to be MPLC because of the absence of conclusive evidence that the minor lesions originated from the major lesions.
In theory, either synchronous or metachronous multiple lung cancers can be explained in two ways: first, a lesion may metastasize from another one and second, the multiple lesions may be unrelated to each other and may all be primary lesions. The term MPLC is used to refer to the latter situation, where 2 or more unrelated primary malignant tumors occur simultaneously or successively in the lungs. In the clinical setting, achieving an accurate differential diagnosis remains a challenge. Clinical manifestations, imaging patterns, histological types, and genetic characteristics should be considered while making a diagnosis. Therefore, in each case, the final diagnosis of MPLC should be made by a multidisciplinary team composed of thoracic surgeons, medical oncologists, radiologists, pathologists, and even geneticists. The rapid development of genotyping techniques for lung cancer diagnosis in recent years may provide stronger support for the diagnosis of MPLC. In our study, one patient had lesions in different ipsilateral lobes. Although the postoperative histopathological type was adenocarcinoma in all lesions, examination of epidermal growth factor receptor (EGFR) gene mutations in various lesions showed that mutation types differed between each lesion. In short, MPLC should be clearly distinguished from stage IV metastatic lung cancer, in order to guide treatment. In this study, the 5-year survival rate of patients with MPLC was as high as 75.8%. In particular, the 5-year survival rate among patients without lymph node metastasis reached 85.7%, similar to that for single primary lung cancer (80.5%). Therefore, surgery should still be considered as the first choice of treatment for MPLC.
Strategies for the surgical treatment of multiple primary lung cancer (MPLC)
Oncologic considerations regarding the extent of surgical resection
In 1995, the clinical trial conducted by Ginsberg established the superiority of lobectomy over sublobectomy for the surgical treatment of lung cancer (9). However, recent retrospective studies showed that if the primary tumor was <2 cm in diameter and no lymph node metastasis was observed, particularly in cases of lesions with ground glass opacity (GGO) on imaging studies, the efficacy of sublobectomy was not inferior to that of lobectomy (10,11). Some ongoing randomized controlled clinical trials in America and Asia are exploring the non-inferiority of lobectomy for this type of small lesion compared to sublobectomy (JCOG 0802/WJOG3406L, CALGB 140503). We hope that the results of these trials would support the therapeutic principle of using sublobectomy to treat early MPLC. Accordingly, sublobectomy might become the primary choice of surgical treatment for MPLC.
Consideration of pulmonary function reserve in determining the extent of surgical resection
Two principles are followed for the surgical resection of MPLC: maximizing tumor removal and maximizing preservation of pulmonary function. For synchronous contralateral MPLC, multistage surgery has been reported most often. The interval between two procedures was approximately 1 month. The reported surgical principles were as follows: (I) the most aggressive central lung cancer should be removed first, and then peripheral lung cancer; (II) larger lesions should be removed first, followed by smaller lesions; (III) a good pulmonary function reserve is required for simultaneous operations; and (IV) the tumors with mediastinal or hilar lymph node metastasis should be removed first, followed by the removal of those without lymph node metastasis. However, we believe that, for synchronous contralateral MPLC, tolerance of the second stage of surgery with respect to pulmonary function should be considered before performing the first stage of surgery. That is, pulmonary function should be preserved as much as possible during the first surgery. The five lobes of the human lung differ in terms of pulmonary function. The left upper lobe is the most important in terms of pulmonary function, followed by the left lower lobe, right lower lobe, right upper lobe, and right middle lobe. Lesions can be classified as either major or minor. For minor lesions located in the main lobes, particularly lesions commonly seen as GGO or small lesions (≤2 cm, sublobectomy, especially anatomic segmentectomy, should be performed if possible. For major lesions (solid, >2 cm) located in subordinate lobes, lobectomy should be performed without hesitation. For other combinations, treatment should be decided according on a case-by-case basis. We believe that multistage surgery is superior to simultaneous surgery and that video-assisted thoracic surgery is preferable. In addition, sublobectomy or lobectomy has little effect on pulmonary function (e.g., middle lobectomy) should be given priority to ensure the safety of the second stage of surgery. In this study, such situations were encountered in 13 cases. Of these, ten patients had synchronous contralateral MPLC, in whom large and small lesions could be distinguished, and sublobectomy was performed to remove the small lesions, followed by lobectomy to remove the contralateral large lesions. Three patients had synchronous contralateral MPLC, in whom sublobectomy could not be performed; thus, multistage lobectomy was performed to remove the bilateral lesions.
The importance of lifelong postoperative follow-up for patients with lung cancer
Thirty percent of patients with early-stage lung cancer died within five years after surgery in cause of recurrence or second primary lung cancer (12). The possible reasons are genetic susceptibility and field cancerization. Among these lung cancer survivors, early detection of the second primary lung cancer or solitary metastasis to lung, followed by appropriate local treatment such as surgery, promotes relatively good long-term efficacy. In this study, metachronous MPLC was detected during long-term regular postoperative follow-up in four cases. The second cancer occurred 24 to 48 months after the initial cancer surgery. Currently, the longest follow-up period is 167 months, with no reported deaths. Therefore, we believe that postoperative follow-up is of important clinical significance for the early diagnosis and treatment of recurrence or second primary lung cancer and for improving patients’ quality of life and long-term prognosis.
The debate on the need for adjuvant chemotherapy
Another currently controversial topic is whether patients should receive adjuvant therapy after surgery. The supporters believe that postoperative chemo could effectively reduce the risks of recurrence and metastasis, which, however, only benefits patients with lymph node metastasis. In contrast, Voltolini et al. (13) and Jung et al. (7) reported that patients with MPLC would not benefit from adjuvant therapy. However, in their studies, patients with lymph node metastasis accounted for a very small proportion of the patient population. Because only five patients who had MPLC in this study also had lymph node metastasis, we could not determine whether postoperative chemo would benefit patient survival. Nevertheless, we believe that the decision to administer adjuvant chemotherapy in cases of MPLC should depend on the T and N staging of the major lesions and should be made in accordance with the existing guidelines for chemotherapy, instead of treating MPLC as metastatic lung cancer.
Limitation
This study was limited by the small number of cases analyzed. Although the database is prospectively maintained, the study was retrospective in nature. Therefore, more cases should be evaluated in prospective studies.
Conclusions
In summary, synchronous and metachronous MPLCs should be staged independently, and surgical treatment should be opted for in cases of early-stage disease. The surgical strategy should be evaluated on the basis of tumor characteristics (early-stage principle) and pulmonary function (safety principle). The data from this study clearly show that surgery for the treatment of MPLC exhibits equivalent efficacy to that for the treatment of single primary lung cancer.
Acknowledgements
Funding: This work was partially supported by Beijing Academic Leaders Program (Grant 2009-2-17), Beijing Natural Science Foundation (Grant 7102029), Capital Medical Developed Research Foundation (Grant 2007-1023), New Scholar Star Program of Ministry of Education (Grant 2011CB504300), and National Basic Research Program of China (973 programs), Specialized Research Foundation for the Doctoral Program of Higher Education (Grant 20130001110108), National Natural Science Foundation (Grant 81301748).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Travis WD, Brambilla E, Müller-Hermelink HK, et al, editors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press, 2004.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Shields TW, Drake CT, Sherrick JC. Bilateral primary bronchogenic carcinoma. J Thorac Cardiovasc Surg 1964;48:401-17. [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Auerbach O, Stout AP, Hammond EC, et al. Multiple primary bronchial carcinomas. Cancer (Philadelphia) 1967;20:699-705. [Crossref] [PubMed]
- Jung EJ, Lee JH, Jeon K, et al. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer 2011;73:237-42. [Crossref] [PubMed]
- Shen KR, Meyers BF, Larner JM, et al. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:290S-305S.
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 1667-8.
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Koike T, Koike T, Yoshiya K, et al. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:372-8. [Crossref] [PubMed]
- Voltolini L, Rapicetta C, Luzzi L, et al. Surgical treatment of synchronous multiple lung cancer located in a different lobe or lung: high survival in node-negative subgroup. Eur J Cardiothorac Surg 2010;37:1198-204. [Crossref] [PubMed]


