Therapeutic value of lymph node dissection for right middle lobe non-small-cell lung cancer
Introduction
The standard treatment for non-small-cell lung cancer (NSCLC) involves lobectomy or pneumonectomy with radical lymphadenectomy (1). However, long-term outcomes following radical mediastinal lymph node dissection (LND) remain controversial, with two major randomized studies producing contradicting results (2,3). Mediastinal LND is essential for accurate staging and improved survival over sampling alone (2), but it does not improve survival in early-stage NSCLC (3). Meanwhile, other retrospective studies have published nodal spread patterns by tumour location (4,5), leading to modified and selective LND becoming increasingly prevalent. Concerns that tumour location is not predictive of nodal metastasis have resulted in the argument that complete systemic mediastinal LND is the only acceptable intervention (6). The pattern of lymphatic drainage from right middle lobe (RML) NSCLCs extends to both superior and inferior mediastinal lymph nodes (LNs); in fact, a high incidence of metastases to these nodal zones has been reported (7,8). Superior mediastinal and #11i LN metastases have been reported to be significant adverse prognostic factors in patients with middle lobe cancer and are associated with each other (9). However, only a few articles have evaluated the therapeutic value of LND during surgical resection of RML NSCLCs. For gastric cancer, Sasako et al. evaluated the therapeutic effect of LND on the basis of incidence of metastasis and 5-year survival rates of those with nodal deposits at a particular station, irrespective of nodal metastasis to other LN stations including para-aortic nodes, to prevent selection bias (10). We therefore applied their methods to evaluate the therapeutic impact of LND on each nodal station or zone for advanced RML NSCLC.
Methods
Patients
We retrospectively studied patients with pN1–2 primary RML NSCLCs. All patients underwent middle lobe resection (at least lobectomy) with thorough mediastinal LND between January 1980 and December 2011. Participants were enrolled at the Aichi Cancer Center Hospital or the Cancer Institute Hospital, Japanese Foundation for Cancer Research. We excluded patients who had undergone pre-operative chemotherapy and radiotherapy and prior LN sampling.
Clinical staging data were obtained by chest and abdominal computed tomography, head magnetic resonance imaging, abdominal ultrasound, bone scintigraphy or positron emission tomography. Tumours were staged according to the TNM classification system (11). Pathological examination was based on the 2004 World Health Organization classification (12). LN location was based on the definitions of the Committee of the International Union against Cancer (13); [#, indicated LN number and (+) and (−) represented positive and negative status of the node, respectively.] The institutional review board of each hospital approved this study without the requirement to obtain patient consent because the identity of each individual patient was concealed.
Method for evaluating the therapeutic value of lymph node dissection (LND)
We used the method described by Sasako et al. to evaluate the therapeutic value of LND according to the index of the benefit for each station (10). The therapeutic index (TI) for every metastasis to a nodal station was calculated by multiplying its frequency by the 5-year survival rate.
Statistical analysis
All data were analysed using SPSS version 17.0 (SPSS Institute Inc., Chicago, Illinois, USA). Sensitivity and specificity were compared using standard formulas. Differences between two groups were calculated using the Mann-Whitney test. Analysis of survival rates was performed using the Kaplan-Meier method and survival rates between patient groups were compared by the log-rank test. A P value of <0.05 was considered to indicate statistical significance.
Results
Descriptive statistics and survival
There were a total of 295 patients with primary RML NSCLCs during the study period. We included the 68 (23.1%) eligible pN1–2 patients (33 men and 35 women) with LN metastases. The mean age of the patients was 68 years and they had confirmed adenocarcinoma (n=53), squamous cell carcinoma (n=11) and other carcinoma (n=4) (Table 1). The pathological nodal statuses were pN0, pN1 and pN2 in 227 (77.0%), 18 (6.1%) and 50 (16.9%) patients, respectively. The median follow-up duration was 1,016 days (range, 42–9,265 days), with the 5-year overall survival (OS) rate significantly higher for pN1 disease than for pN2 disease (58.3% vs. 28.6%; P=0.02).
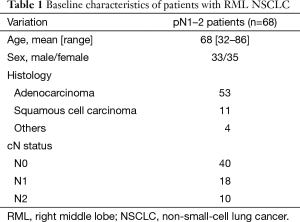
Full table
Node metastasis and spread pattern
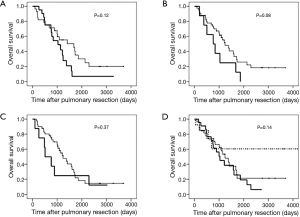
Table 2 summarizes the frequency of nodal involvement by station. Most were N1 nodes (#10–#14, 80.9%), and the numbers of superior (#2R–#4R, 48.5%) and inferior (#7–#9, 58.8%) mediastinal nodes were comparable. Skip node N2 (SN2) metastases were evident in 13 patients (26.0%), of whom 8 (61.5%) had visceral pleural invasion; the upper zone (UZ) and subcarinal zone (SCZ) were equally involved (P=0.69). For pN1 (n=18), the most frequent site for metastasis was #12m–#14 (15 cases, 83.3%), followed by #10 (2 cases, 11.1%) (P<0.01); the lobar nodes (#11s and #11i) were rarely involved (5.6% and 0%, respectively). For pN2 (n=50), the frequency of SCZ and UZ involvement was the same [39 (78.0%) patients vs. 33 (66.0%) patients; P=0.18]. Combined metastases to UZ and SCZ occurred in 23 patients (46.0%), whereas lower zone (LZ) metastases were noted in only 4 patients (8.0%). The results for the analysis of pN2 via #10 (n=24; P=0.34; Figure 1A), #11s (n=10; P=0.62; Figure 1B), #11i (n=9; P=1.00; Figure 1C) and #12m (n=36; P=0.35; Figure 1D) showed no significant difference between UZ and LZ among each N1 node category.
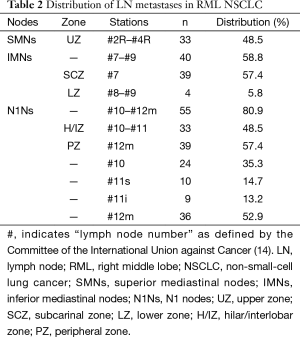
Full table
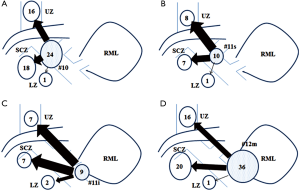
Predictive benefit of lymph node (LN) involvement in middle lobe non-small-cell lung cancer (NSCLC)
The 5-year OS rates of pN2 disease by LN zones were 10.5% (n=33) for UZ, 24.7% (n=39) for SCZ and 6.5% (n=23) for both UZ and SCZ. The 5-year OS rate was not significantly better for SN2 metastases compared with that for non-SN2 metastases (P=0.30). The 5-year OS rates were 38.9% for SN2 single-station (SS) metastases [N1(−)N2(+) (n=9)], 36.0% for non-SN2-SS metastases [N1(+)N2(+) (n=18)], 0.9% for non-SN2 multiple-station (MS) metastases [N1(+)N2(+) (n=19)] and 0% for SN2-MS metastases [N1(−)N2(+) (n=4)]. Comparison of most of these 5-year OS rates were not significant (P=0.06); however, a significant difference was noted between SS and MS 5-year OS rates (37.7% vs. 6.5%; P=0.01) (Figure 2).
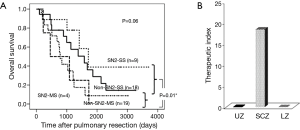
Metastasis and 5-year survival rate
We explored the distribution of pN2 (n=50) and prognosis by N1 category. The #10, #11s, #11i and #12u populations accounted for 91.7%, 90.0%, 100% and 63.9%, respectively. Cases with pN2 metastases were divided according to N1 locations as N1(−)N2(+) and N1(+)N2(+). Cases with pN1–2 metastases were divided according to the #12m location as N1(+)N2(−), N1(−)N2(+) and N1(+)N2(+). The 5-year OS rates according to the divisions of pN2 and pN1–2 metastases are summarized in Figure 3.
Therapeutic value of lymph node dissection (LND) and associated clinicopathological features
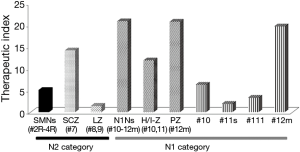
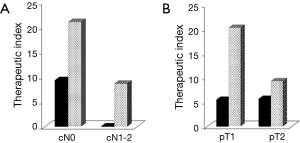
SCZ (24.7%) and LZ (25.0%) nodal involvements had equivalent 5-year OS rates to hilar/interlobar zone (H/IZ) involvement (24.5%). The 5-year OS was best for the peripheral zone (PZ, 12m–#14) (36.3%), and worst for UZ (10.5%). Zone-specific prognostic tendency was identified by the distance of the primary tumour from the lymphatic location. The benefits of LND are summarized in Figure 4. The TI for SCZ (14.2) was superior to that for H/IZ (11.9), 2.8-fold higher than that for UZ and 9.7-fold higher than that for LZ. In pN2 NSCLC, the TI for the H/IZ positions #10, #11s and #11i fluctuated from 1.9 to 6.3 (Figure 4). Regional LNs defined as N2 status (SCZ, #7) by the American Joint Committee on Cancer classification system had a higher TI that than for those designated as N1 (H/IZ, #10, #11s, #11i). Furthermore, the TI for SCZ in patients with SN2 metastases was 1.3-fold higher than that in patients with pN2, but there was no expectation for UZ and LZ involvement (Figures 2B,4). The correlation between the clinicopathological features and TI for the pN2 zone [left UZ (LUZ) and SCZ] is shown in Figure 5. According to TI, LND for LUZ was not efficient for patients with clinical N1–2 (cN1–2) disease (Figure 5A); therefore, the patients were divided into two groups by tumour size. Although a difference in tumour size did not influence the TI for UZ, the TI for SCZ in patients with a pT1 tumour was 2.2-fold higher than that in patients with a pT2 tumour (Figure 5B).
Discussion
The reported frequency of metastasis for RML NSCLC is inconsistent. RML NSCLCs have been reported to be more likely to have N1 disease (33%) and pN2 disease (15%), with the latter result being consistent with ours (14). Furthermore, in the study by Riquet et al., pN1 and pN2 disease occurred in 6.3% and 18.8% of lobotomized patients, respectively (7). However, our results support Yamanaka et al., who reported frequencies of 4.5% (1/22) and 40.9% (9/22), respectively (15). However, it is important to note that most of these analyses have been confounded by limited data (7,14,15).
We have described three points about nodal metastases from middle lobe cancer. First, UZ and #11i metastases present significant adverse prognostic factors in patients with middle lobe lung cancer. Second, #11i metastases may result from mediastinal metastases (9). This is supported by our results that show rare involvement of the interlobar nodes of the middle lobe. Third, the prognostic impact must be different from that for other lobes (9). The findings of our study were consistent with those of other reports, with the prognosis being significantly worse for patients with mediastinal LN metastases than for those with N1 nodes only.
When examining whether the path of LN spread affected prognosis, no association existed for mediastinal LN metastases without H/IZ LN involvement, but a tendency did exist for #12. Although H/IZ and PZ belong to the same N1 group, the TI for the former was less. The effect of LND gradually weakened in N1 disease in the order #11i, #11s, #10 and #12m, with a three-fold difference between #10 and #12m.
In the development of an LN mapping system, early controversy centred on whether to classify tracheobronchial #10 nodes as N1 or N2 (16). LNs around the main bronchus have been designated as intermediate, with no distinction between N1 and N2 nodes (17). In this report, the TI for #10 was comparable to that for UZ, but was lower than that for SCZ. Moreover, the incidence of #11s metastases (5.6%) was similar to that reported in a previous study (9). We hypothesized that #11i metastases were retrograde because antegrade drainage to the superior mediastinum from mediastinal metastasis was disturbed. Although classified as pN1 nodes, H/IZ nodes may be handled by surgeons as pN2 disease.
Sasako et al. commented that their method attempted to determine the actual benefit of LND and that it circumvented the phenomenon of stage migration in gastric cancers (10). Although ipsilateral hilar and standard mediastinal LND is known to be the standard method of LND for RML NSCLCs, we intend to additionally quantify the role of extensive LND, specifically the priority LN stations or zones during middle lobe resection, using the TI described approximately 20 years ago. However, we considered that the TI was available to evaluate the classification and effectiveness of LND regardless of the number of LN metastases.
We have reported that UZ and SCZ nodes are major metastatic sites and that prognosis is comparable to that of multi-level N2 middle lobe cancer when superior mediastinal LNs is involved (9). Here we observed that the TI was higher for SCZ than for UZ or LZ, implying that precise pathological staging was probably more important than LND, perhaps because these generally need intensive treatment. Consistent with our previous report, patients with cN1–2 and pN2 middle lobe cancer gained little benefit from LND (18). Furthermore, the TIs among pN2 patients with pT1 and pT2 UZ disease were similar, suggesting that the pathological node status was more important than the tumour size as an adverse prognostic factor and that precise pathological staging was again more important than LND. We found no benefit from adjuvant chemotherapy. When planning LND for NSCLC, surgeons must know the expected benefit by nodal station or zone to ensure optimal LND and benefit from adjuvant chemotherapy. When considering which zone to prioritize, the frequencies were the same between UZ and SCZ; surgeons may prefer the latter, based on previous reports. SCZ nodes are more common in RML and lower lobe malignancies (19) and are generally grouped together (20). In addition, RML cancers metastasize to both UZ and SCZ with equivalent frequency (4,14,21). RML malignancies with LNs at the sump location can have metastatic involvement (16), with most LN drainage to the superior rather than the inferior LNs (22). Yamanaka have commented that the pattern of lymph drainage from the middle lobe to the interlobar LNs is similar to that in the basal segments of the lower lobe (15).
Next, we considered whether selective lymphadenectomy was possible. Good therapeutic effectiveness was obtained for SCZ and H/IZ, with benefit from UZ LND being approximately one-third of that from SCZ LND. Although the numerical value was low for pre-operatively diagnosed cN1–2 or pT2 stage RML NSCLC, we considered it sufficient justification for LND. Pre-operative manipulation of patient selection to authentic pN0 by multi-detector row computed tomography (MDCT) was required for selective LND. We previously emphasized the correlation between LN metastases and mediastinal tumour size on MDCT despite evidence from retrospective studies and suggested the need for this manipulation (23). Therefore, our method of cN0 patient selection by MDCT was necessary for selective LND.
The #12u node dissection in RML lobectomy is technically more challenging and requires longer operative time, particularly when performed thoracoscopically. Considering that #12u is usually adjacent to #11s, en-bloc resection is often necessary while pulling the superior truncus artery and superior pulmonary vein. In Japan, the incidence of #12u involvement was 9.2% (14/152) in a study that recommended routine dissection in patients with carcinoma of RML, right lower lobe or left lower lobe (24). The TI of LND was calculated as 4.8 in that report, which was compatible with that for UZ and #11s nodes in our study. Although we found no reports regarding #12u metastases confined to the middle lobe, there is plausible evidence for #12u LND in this analysis. Further investigation is necessary to clarify the role of such dissection in RML NSCLCs.
Another important result was related to SN2 metastases in RML, which had equal incidences between UZ and SCZ involvement. The most favourable prognosis was for pN2 with SN2-SS metastases (38.9%), followed by non-SN2 metastases (23.0%). The 5-year OS rate of patients with SN2-MS metastases and involvement of both UZ and SCZ was 0% (n=4). Although no significant difference was found among the three groups, stratified prognosis was identified. The significance of SCZ dissection was higher for patients with SN2 metastases than for those with non-SN2 metastases. In addition, among patients with SN2 UZ involvement, six out of eight survived for less than 5 years post-operatively, whereas the remaining two survived for more than 3 years (mean, 1,216 days). This may be related to the fact that metastases to higher-position nodes are not searched during surgery, which is compatible with previous reports (10). Visceral pleural invasion was not applied to the correlation for middle lobe SN2 metastases because we included pN2 patients, not cIA NSCLC (25).
This study has some limitations, including its retrospective design, small sample size and inclusion of cases from the 1980s. To avoid institutional bias, this study was undertaken at two specialist centres in a cohort with different therapeutic strategies. Moreover, the study required lobectomy with thorough lymphadenectomy as the standard pulmonary resection, thereby precluding generalization. To provide valid prognostic data, a prospective study has been planned.
In conclusion, nodal H/IZ involvement in RML NSCLC had a tendency towards unfavourable prognosis. Interlobar node involvement was rare in comparison with hilar and lobar nodal metastases for middle lobe N1 NSCLC. The benefit from H/IZ dissection was intermediate to that from UZ and SCZ, whereas TI revealed greater effectiveness from SCZ over UZ dissection in pN2 middle lobe NSCLC, which was similar for SN2 metastases. H/IZ involvement, therefore, had a key role and mediastinal SCZ dissection should be prioritized over UZ dissection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555-72. [PubMed]
- Wu Y, Huang ZF, Wang SY, et al. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002;36:1-6. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Asamura H, Nakayama H, Kondo H, et al. Lobe-specific extent of systematic lymph node dissection for non-small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg 1999;117:1102-11. [Crossref] [PubMed]
- Shimada Y, Saji H, Kakihana M, et al. Retrospective analysis of nodal spread patterns according to tumor location in pathological N2 non-small cell lung cancer. World J Surg 2012;36:2865-71. [Crossref] [PubMed]
- Riquet M, Rivera C, Pricopi C, et al. Is the lymphatic drainage of lung cancer lobe-specific? A surgical appraisal. Eur J Cardiothorac Surg 2015;47:543-9. [Crossref] [PubMed]
- Riquet M, Dupont P, Hidden G, et al. Lymphatic drainage of the middle lobe of the adult lung. Surg Radiol Anat 1990;12:231-3. [Crossref] [PubMed]
- Hata E, Hayakawa K, Miyamoto H, et al. Rationale for extended lymphadenectomy for lung cancer. Theor Surg 1990;5:19-25.
- Sakao Y, Okumura S, Mingyon M, et al. The impact of superior mediastinal lymph node metastases on prognosis in non-small cell lung cancer located in the right middle lobe. J Thorac Oncol 2011;6:494-9. [Crossref] [PubMed]
- Sasako M, McCulloch P, Kinoshita T, et al. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 1995;82:346-51. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol 2005;40:90-7. [Crossref] [PubMed]
- Blackwell W. International union against cancer. In: Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th Edition. New York: Wiley-Liss, 2009.
- Cerfolio RJ, Bryant AS. Distribution and likelihood of lymph node metastasis based on the lobar location of nonsmall-cell lung cancer. Ann Thorac Surg 2006;81:1969-73; discussion 1973.
- Yamanaka A, Hirai T, Takahashi A, et al. Interlobar lymph node metastases according to primary tumor location in lung cancer. Lung Cancer 2002;35:257-61. [Crossref] [PubMed]
- Kim AW. Lymph node drainage patterns and micrometastasis in lung cancer. Semin Thorac Cardiovasc Surg 2009;21:298-308. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Border between N1 and N2 stations in lung carcinoma: lessons from lymph node metastatic patterns of lower lobe tumors. J Thorac Cardiovasc Surg 2005;129:825-30. [Crossref] [PubMed]
- Sakao Y, Okumura S, Mun M, et al. Prognostic heterogeneity in multilevel N2 non-small cell lung cancer patients: importance of lymphadenopathy and occult intrapulmonary metastases. Ann Thorac Surg 2010;89:1060-3. [Crossref] [PubMed]
- Nohl-Oser HC. An investigation of the anatomy of the lymphatic drainage of the lungs as shown by the lymphatic spread of bronchial carcinoma. Ann R Coll Surg Engl 1972;51:157-76. [PubMed]
- Watanabe S, Suzuki K, Asamura H. Superior and basal segment lung cancers in the lower lobe have different lymph node metastatic pathways and prognosis. Ann Thorac Surg 2008;85:1026-31. [Crossref] [PubMed]
- Kotoulas CS, Foroulis CN, Kostikas K, et al. Involvement of lymphatic metastatic spread in non-small cell lung cancer accordingly to the primary cancer location. Lung Cancer 2004;44:183-91. [Crossref] [PubMed]
- Shields TW. Lymphatics of the lungs. In: Shields TH, Locicero J III, Reed CE, editors. General Thoracic Surgery. Philadelphia: Lippincott Williams & Wilkins, 2009:87-101.
- Sakao Y, Kuroda H, Mun M, et al. Prognostic significance of tumor size of small lung adenocarcinomas evaluated with mediastinal window settings on computed tomography. PLoS One 2014;9:e110305. [Crossref] [PubMed]
- Sato M, Saito Y, Aikawa H, et al. Involvement of the #12u lymph nodes around the upper lobe bronchi in patients with lung cancer of the right middle lobe, right lower lobe, or left lower lobe. Surg Today 1999;29:238-42. [Crossref] [PubMed]
- Gorai A, Sakao Y, Kuroda H, et al. The clinicopathological features associated with skip N2 metastases in patients with clinical stage IA non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;47:653-8. [Crossref] [PubMed]


