Clinical results of sublobar resection versus lobectomy or more extensive resection for lung cancer patients with idiopathic pulmonary fibrosis
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive parenchymal lung disease with a poor prognosis and of unknown cause (1,2). The median survival is reported to be 2–4 years from the time of diagnosis (1). IPF is associated with an increased risk of lung cancer. The prevalence of lung cancer in patients with IPF is high, ranging from 4.8% to 48% (3-8). In lung cancer patients, the postoperative pulmonary morbidity and mortality of patients with IPF are found to be higher than in those without IPF (3,6,8-10). Acute postoperative exacerbation of IPF (AEIPF) is a fatal complication that can occur after lung resection. The mortality of AEIPF is known to be very high, ranging from 80–100% (6).
The optimal surgical strategy for lung cancer in patients with IPF remains undetermined. Partial resection might reduce the occurrence of morbidity and in-hospital mortality but might also increase the recurrence rate and reduce the survival rate compared with a lobectomy or more extensive resection. This study aimed to compare the clinical results of a sublobar resection versus a lobectomy or more extensive resection for lung cancer in patients with IPF.
Methods
Patients
From January 1995 to December 2012, 80 consecutive patients with simultaneous non-small cell lung cancer and IPF were treated surgically at Asan Medical Center (AMC). The medical records of these cases were reviewed retrospectively. Data acquisition and analyses were approved by the institutional review board of AMC. The medical records contained the patient characteristics, operative procedures, pathological diagnoses, and follow-up data.
The sublobar resection group included patients who underwent a sublobar pulmonary resection and the lobar resection group included patients who underwent a lobectomy or more extensive resection for their lung cancer. The diagnosis of IPF was defined according to the International Consensus Statement of the American Thoracic Society/European Respiratory Society (11). Exclusion criteria included connective tissue disease, occupational or environmental lung disease, previous radiation therapy or metastatic disease of the lung, and any history of ingestion of a drug or an agent known to cause pulmonary fibrosis.
Operative procedures were selected based on the operating surgeon’s preference.
Statistical analyses
All descriptive statistics were expressed as means ± standard deviation (SD) for continuous variables. Categorical data were analyzed with Fisher’s exact test and linear by linear association. Continuous data were compared using Student’s t-test and the Mann–Whitney U-test. Predictors of overall survival and recurrence-free survival were evaluated. Statistical analyses included Kaplan–Meier estimates of survival, log-rank tests of survival differences, and multivariate Cox proportional hazards models. Differences were considered to be statistically significant with P values <0.05. Statistical analyses were performed using SPSS statistical software version 21.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
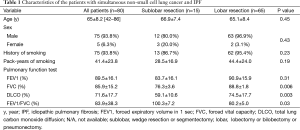
The characteristics of the study cohort are listed in Table 1. The mean patient age was 65 years (range, 42–86 years) and most were male (M:F =75:5). In the sublobar resection group, 12 patients were male and 3 were female, whereas in the lobar resection group, 63 patients were male and 2 were female (P=0.43). A total of 75 (93.8%) patients had a history of smoking at 41.4 mean pack-years. There were no significant differences in the history of smoking or pack-years of smoking between the two groups (P=0.23 and P=0.19, respectively). For the preoperative PFT, the mean FEV1 was 89.5% of the predicted value, the FVC was 85.9%, the DLco was 71.6%, and the FEV1/FVC (%) ratio was 83.9%. Regarding the preoperative PFT, the mean FEV1 (%) was not significantly different between the study groups (83.7% in the sublobar resection group and 90.9% in the lobar group; P=0.31), FVC (%) was significantly lower in the sublobar resection group than in the lobar resection group (76.3% vs. 88.8%; P=0.006), DLCO (%) was significantly lower in the sublobar resection group than in the lobar resection group (59.1% vs. 74.5%; P=0.003), and FEV1/FVC (%) was significantly higher in the sublobar resection group than in the lobar resection group (100.3% vs. 80.2%; P=0.03).
Full table
Operative procedures
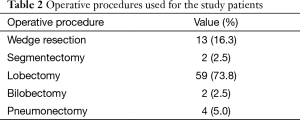
Lobectomy was the most frequent operative procedure (n=59, 73.8%) with sublobar resections performed in 15 (18.8%) of the study patients (Table 2).
Full table
Perioperative variables
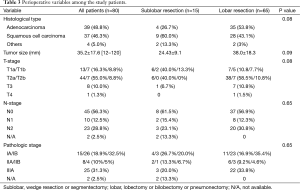
The rate incidence was similar by histology between adenocarcinoma (n=39, 48.8%) and squamous cell carcinoma (n=37, 46.3%) with no significant difference between the two groups (P=0.08). The mean tumor size was 35.2 mm (range, 12–120 mm) with no significant difference between the two groups (P=0.09). In terms of postoperative pathology, the most frequent T-stage was T2a found in 44 (55.0%) patients, the most frequent N-stage was N0 seen in 45 (56.3%) patients, and stage I, II, and IIIA tumors occurred in 41 (51.4%), 12 (15%), and 25 (31.3%) patients, respectively. Exact pathologic staging was not possible in two patients of the sublobar resection group because lymph node dissection or sampling was not performed. There were no significant differences in the postoperative T-stage, N-stage, or pathological stage between the two study groups (P=0.08, P=0.65, and P=0.65, respectively; Table 3).
Full table
Postoperative results
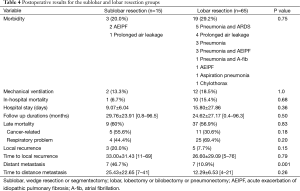
The sublobar resection group showed less morbidity after pulmonary resection than the lobar resection group, but this difference was not statistically significant (20.0% vs. 29.2%; P=0.75). A total of 2 and 12 patients in the sublobar and lobar resection groups received mechanical ventilation, respectively, including 1 and 10 patients who died (13.3% vs. 18.5%; P=1.0). All in-hospital mortalities were related to respiratory problems. The sublobar resection group showed less in-hospital mortalities after pulmonary resection than the lobar resection group but this difference was not statistically significant (6.7% vs. 15.4%; P=0.68). The sublobar resection group had fewer hospital stays after pulmonary resection than the lobar resection group, but this difference was not statistically significant (9.07 vs. 15.80 days; P=0.36). The mean follow-up duration was 29.76±23.91 months (range, 0.8–96.5 months) in the sublobar resection group and 24.62±27.17 months (range, 0.4–96.3 months) in the lobar resection group. Regarding late mortality, we detected no significant difference between the sublobar and lobar resection groups (60.0% vs. 56.9%; P=0.83). Cancer-related deaths were more frequent in the sublobar resection group (55.6% vs. 30.6%; P=0.18), while deaths related to respiratory problems were more frequent in the lobar resection group (44.4% vs. 69.4%; P=0.20); however, these differences were also not statistically significant. Local recurrence was more frequent in the sublobar resection group than in the lobar resection group, but this again was not significant (20.0% vs. 7.7%; P=0.15). The mean time to local recurrence was 33.00±31.43 months (range, 11–69 months) in the sublobar resection group and 26.60±29.09 months (range, 5–76 months) in the lobar resection group (P=0.79). Distant metastasis was significantly more frequent in the sublobar resection group than in the lobar resection group (46.7% vs. 10.9%; P=0.001). The mean time to distant metastasis was 25.43±22.65 months (range, 7–41 months) in the sublobar resection group and 12.29±6.53 months (range, 4–21 months) in the lobar resection group (P=0.26; Table 4).
Full table
Survival analysis
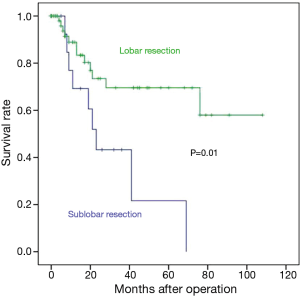
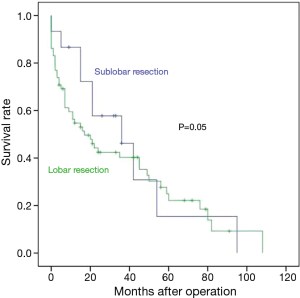
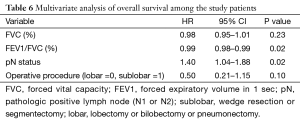
The one-, three-, and five-year recurrence-free survival rates in the sublobar and lobar resection groups were 69.2% vs. 88.9%, 43.3% vs. 69.6%, and 21.6% vs. 69.6%, respectively. Recurrence-free survival was significantly longer in the lobar resection group (P=0.01; Figure 1). The one-, three-, and five-year overall survival rates in the sublobar and lobar resection groups were 86.7% vs. 54.7%, 46.2% vs. 40.3%, and 15.4% vs. 22.2%, respectively. There was no difference in the rate of overall survival between the two groups (P=0.05; Figure 2). By multivariate analysis, the pathologically positive lymph node (N1 or N2) status (hazard ratio =2.19; 95% CI: 1.14–4.20; P=0.02) and sublobar resection (hazard ratio =4.74; 95% CI: 1.12–20.06; P=0.03) were significant risk factors for predicting recurrence-free survival (Table 5). By multivariate analysis, a pathologically positive lymph node (N1 or N2) status (hazard ratio =1.40; 95% CI: 1.04–1.88; P=0.02) and the FEV1/FVC (hazard ratio =0.99; 95% CI: 0.98–0.99; P=0.02) were found to be significant risk factors that could predict overall survival (Table 6).

Full table
Full table
Discussion
IPF is associated with an increased risk of lung cancer, with a relative risk of 7.0–14.0 (compared with the general population) (7). Approximately 2% to 4% of lung cancer patients have IPF (2). It is difficult to treat these patients because of the poor prognosis of IPF itself (6,8,12), and the high morbidity and mortality rates after lung resection (1-3,6-16). In lung cancer patients with IPF, the postoperative pulmonary morbidity and mortality rates after lung resection have been reported to range from 33.3–54% and 7.4–18.2%, respectively (3,6-16).
Yano et al. have previously reviewed five Japanese studies published from 1992 through 1998 on surgery for lung cancer patients with IPF. These authors found a high rate of acute exacerbation in pneumonectomy and no cases of acute exacerbation in wedge resection (17). Okamoto et al. reported a higher incidence of IPF exacerbation in patients who had undergone lobectomy or pneumonectomy, and no occurrence in those patients who had undergone partial or segmental resection (10). Yano et al. have also reported that the resected lung volume or the degree of operative insult might be related to postoperative acute exacerbation (9). Numerous studies to date have suggested that greater operative insult may also be associated with an increased risk of acute exacerbation and postoperative mortality (1,3,6,7,9,10,14,17). Koizumi et al. compared three surgical approaches—posterolateral thoracotomy, muscle sparing thoracotomy, and video-assisted thoracic surgery—and reported that video-assisted thoracic surgery did not prevent acute exacerbation of IPF (13). The advantages of partial resection may depend upon many factors, such as a smaller lung resection volume, shorter anesthesia time, shorter operation time, and less bleeding or surgical stress (9). Low oxygen inhalation, the use of steroids, limited surgery, avoidance of hyperinflation of the lung during operation, and prevention of postoperative pneumonia by prophylactic antibiotic therapy have been used to prevent acute exacerbation of IPF after lung resection (6). In our present study of lung cancer patients with IPF, no statistically significant differences in the rates of incidence were detected between the two study groups; however, the sublobar resection group showed fewer morbidities than the lobar resection group (20.0% vs. 29.2%; P=0.75), and also a lower level of in-hospital mortalities than the lobar resection group (6.7% vs. 15.4%; P=0.68).
Patients with IPF experience a high rate of occurrence of new malignancies but the reason for this phenomenon is unknown. The generally accepted mechanism is that inflammatory cells produce various types of cytokines that can stimulate the proliferation of epithelial cells, a process that results in carcinoma formation along with oncogene activation (12). Some researchers have argued that considering the high recurrence rate and poor prognosis for this patient population, a limited resection is acceptable if it can be achieved with an adequate margin (7,12). Sato et al. reported a five-year survival rate of 29.2% for wedge resection, 60.0% for segmentectomy, and 68.6% for lobectomy. Additionally, for deaths related to respiratory problems, these authors reported an odds ratio for wedge resection versus lobectomy of 0.35 (P=0.15), and for segmentectomy versus lobectomy of 0.80 (P=0.64). Finally, they commented that wedge resection patients were less likely to develop AEIPF, but had a higher cancer recurrence rate than the lobectomy group (18). In contrast, Watanabe et al. reported that the extent of lung resection had no effect long-term outcomes for lung cancer among patients with IPF. They compared sublobar resection with lobectomy, and reported five-year survival rates for wedge resection, segmentectomy, and lobectomy of 62.4%, 50.0%, and 53.6%, respectively (P=0.93) (6).
In our present study of lung cancer patients with IPF, distant metastasis after lung resection was significantly more frequent in the sublobar resection group (46.7% vs. 10.9%; P=0.001), but the incidence of local recurrence was not significantly different between our two study groups (20.0% vs. 7.7%; P=0.15). Regarding late mortality after lung resection, cancer-related deaths were found to be more frequent in the sublobar resection group (55.6% vs. 30.6%; P=0.18), whilst deaths related to respiratory problems showed a higher incidence in the lobar resection group (44.4% vs. 69.4%; P=0.20), although these differences were not statistically significant.
Study limitations
Our study had several limitations. First, it was limited to small patients particularly in the sublobar resection group. A lot of variables did not reach statistical significance presumably due to the small number. Second, it was a retrospective study. We could not include more detailed information regarding the operative procedures throughout the study period. Third, the selection criteria regarding operative procedure were surgeon’s preferences. There are inherent selection biases. Generally speaking, sublobar resection group has a more compromised cardiopulmonary function or a severer pulmonary fibrosis. But, we could not exactly acquire the selection criteria regarding operative procedure. To further validate our results, future prospective analyses of a larger group of patients are needed.
Conclusions
In conclusion, although not statistically significant, a sublobar resection results in less in-hospital mortality than a lobar resection for lung cancer patients with IPF. Although more cancer related deaths arise following a sublobar resection, there is no significant difference in overall survival compared with lobar resection. Therefore, a sublobar resection may be another therapeutic option for lung cancer patients with IPF.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ghatol A, Ruhl AP, Danoff SK. Exacerbations in idiopathic pulmonary fibrosis triggered by pulmonary and nonpulmonary surgery: a case series and comprehensive review of the literature. Lung 2012;190:373-80. [Crossref] [PubMed]
- Yüksel M, Ozyurtkan MO, Bostanci K, et al. Acute exacerbation of interstitial fibrosis after pulmonary resection. Ann Thorac Surg 2006;82:336-8. [Crossref] [PubMed]
- Kushibe K, Kawaguchi T, Takahama M, et al. Operative indications for lung cancer with idiopathic pulmonary fibrosis. Thorac Cardiovasc Surg 2007;55:505-8. [Crossref] [PubMed]
- Hubbard R, Venn A, Lewis S, et al. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000;161:5-8. [Crossref] [PubMed]
- Ozawa Y, Suda T, Naito T, et al. Cumulative incidence of and predictive factors for lung cancer in IPF. Respirology 2009;14:723-8. [Crossref] [PubMed]
- Watanabe A, Higami T, Ohori S, et al. Is lung cancer resection indicated in patients with idiopathic pulmonary fibrosis? J Thorac Cardiovasc Surg 2008;136:1357-63, 1363.
- Watanabe A, Miyajima M, Mishina T, et al. Surgical treatment for primary lung cancer combined with idiopathic pulmonary fibrosis. Gen Thorac Cardiovasc Surg 2013;61:254-61. [Crossref] [PubMed]
- Kawasaki H, Nagai K, Yoshida J, et al. Postoperative morbidity, mortality, and survival in lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol 2002;81:33-7. [Crossref] [PubMed]
- Yano M, Sasaki H, Moriyama S, et al. Post-operative acute exacerbation of pulmonary fibrosis in lung cancer patients undergoing lung resection. Interact Cardiovasc Thorac Surg 2012;14:146-50. [Crossref] [PubMed]
- Okamoto T, Gotoh M, Masuya D, et al. Clinical analysis of interstitial pneumonia after surgery for lung cancer. Jpn J Thorac Cardiovasc Surg 2004;52:323-9. [Crossref] [PubMed]
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646-64. [Crossref] [PubMed]
- Fujimoto T, Okazaki T, Matsukura T, et al. Operation for lung cancer in patients with idiopathic pulmonary fibrosis: surgical contraindication? Ann Thorac Surg 2003;76:1674-8; discussion 1679.
- Koizumi K, Hirata T, Hirai K, et al. Surgical treatment of lung cancer combined with interstitial pneumonia: the effect of surgical approach on postoperative acute exacerbation. Ann Thorac Cardiovasc Surg 2004;10:340-6. [PubMed]
- Shintani Y, Ohta M, Iwasaki T, et al. Predictive factors for postoperative acute exacerbation of interstitial pneumonia combined with lung cancer. Gen Thorac Cardiovasc Surg 2010;58:182-5. [Crossref] [PubMed]
- Chiyo M, Sekine Y, Iwata T, et al. Impact of interstitial lung disease on surgical morbidity and mortality for lung cancer: analyses of short-term and long-term outcomes. J Thorac Cardiovasc Surg 2003;126:1141-6. [Crossref] [PubMed]
- Kumar P, Goldstraw P, Yamada K, et al. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg 2003;125:1321-7. [Crossref] [PubMed]
- Yano T, Koga T, Ninomiya S, et al. A review of Japanese literatures concerning surgery for lung cancer with idiopathic interstitial pneumonia. Kyobu Geka 2002;55:131-3; discussion 133-4. [PubMed]
- Sato T, Watanabe A, Kondo H, et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 2015;149:64-9, 70. [Crossref] [PubMed]


