Where do we go from here? Reappraising the data on anticoagulation in pulmonary arterial hypertension
The utility of anticoagulation in pulmonary arterial hypertension (PAH) has long been a source of debate between experts in the field (1,2). There is strong pathophysiologic rationale for this type of therapy, as numerous studies have identified in situ thrombosis and a pro-coagulant milieu in PAH (3-9). Unfortunately, a paucity of randomized controlled studies has left practicing clinicians relying on a handful of observational investigations to guide practice patterns (1,2,10,11). Prior to publication of the Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL) registry anticoagulation report, there were 9 cohort studies [2 prospective (12,13) and 7 retrospective (6,14-19)] specifically addressing the question of anticoagulation in PAH. Overall, the data favored anticoagulation, with a 31% reduction in mortality described in a meta-analysis and systematic review—although with concern for publication bias (10,20). In line with this finding, international guidelines continue to recommend use of anticoagulation in idiopathic pulmonary artery hypertension (iPAH) but recognize the scarcity of supportive trials (9,21,22). Guidance for anticoagulation in connective tissue disease-associated PAH (CTD-PAH) remains even more elusive (14). Only 2 (13,14) of 9 studies included in the meta-analysis by Caldeira and colleagues (10) explicitly address CTD-PAH and they demonstrate incongruous findings.
The 2015 Circulation publication describing analysis of anticoagulation in the REVEAL registry increases the clinical uncertainty surrounding this question (23). This United States based-retrospective registry assessed the effect of warfarin treatment on survival in patients with iPAH and systemic sclerosis-associated PAH (SSc-PAH). iPAH and SSc-PAH patients who started warfarin after enrollment into the registry were matched with patients never on warfarin based on enrollment center, etiology and diagnosis status. In the iPAH cohort, there was no significant survival benefit observed in the unadjusted or REVEAL risk score (24) -adjusted analyses. The SSc-PAH warfarin cohort had increased mortality with an unadjusted hazard ratio of 2.03 (P=0.03), decreased to an insignificant 1.6 when adjusted for the risk score. An adjusted time-varying cox proportional model was performed on the unmatched sample to account for frequent warfarin starts and stops. The iPAH warfarin cohort still had no significant survival benefit, but interestingly the hazard ratio dropped below 1. The SSc-PAH group maintained a similar trend toward increased mortality in warfarin users.
The stir generated by this publication is amplified in the setting of a similar registry study, the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) registry, published almost two years earlier with discordant results (14). COMPERA also examined anticoagulation in PAH, with analysis of both iPAH and SSc-PAH cohorts, in a mostly German population. Similar to REVEAL, COMPERA found no mortality benefit with a trend toward decreased survival in the SSc-PAH group treated with anticoagulation. On the other hand, both matched and unmatched survival analyses demonstrated a statistically significant mortality benefit in iPAH anticoagulation users. In the matched analysis, the survival difference was 4%, 11% and 12% at 1, 2, and 3 years follow-up, respectively (P=0.017).
The results of the COMPERA analysis overall align with the previously published studies (10)—so what is different about these seemingly similar registries that may account for the novel findings in regards to iPAH and anticoagulation in REVEAL? Focusing on iPAH, both studies enrolled a similar number of patients and the matched-pair analyses were comparable in size (REVEAL: 144 pairs, COMPERA: 183 pairs). The mean age of patients in the iPAH cohorts is quite different: those in the COMPERA registry are approximately 20 years older than the comparison REVEAL group (72 vs. ~50 years of age). COMPERA had 56% female participants, compared to 80% in REVEAL. The COMPERA iPAH patients also appear sicker, with a lower baseline 6-minute walk distance (6MWD) and worse World Health Organization (WHO) functional class: 96% of patients with class III/IV vs. only ~55% in the REVEAL iPAH cohort. Comorbidities were comparable between iPAH patients on warfarin compared to those not on warfarin in the REVEAL study, but have not been reported from COMPERA, limiting direct evaluation (14,25). One possible explanation is that the older, more morbid and male iPAH participants in the COMPERA study represent a changing demographic admixed with the more stereotypical iPAH patients described in prior studies and the REVEAL registry population (26). There is great potential that this differing demographic has unidentified comorbidities, such as arrhythmias, that could explain the mortality benefit with anticoagulation seen in COMPERA and not REVEAL (27,28). Arguing against this hypothesis, however, is the fact that the REVEAL registry population is actually more similar to the previously published studies that did find survival benefit with anticoagulation (10).
There are also noteworthy differences in the PAH-targeted therapies employed in the two registries, reflecting variance in clinical practice patterns between US and European experts (29). In the REVEAL iPAH cohort, 58% of patients on warfarin and 32% not on warfarin received a prostacyclin analogue (intravenous, subcutaneous, inhaled or oral). This contrasts with the medications reported in COMPERA, where far fewer patients were reported to be on prostacyclin analogues. Prostacyclin therapy not only provides vasodilation in PAH, but has also been shown to alter hemostatic and platelet pathways, possible decreasing the in situ thrombosis and hypercoagulability observed in patients with PAH (3,7,30-32). Of note, bleeding risks are increased in patients with iPAH, CTD-PAH and chronic thromboembolic pulmonary hypertension when vitamin K antagonists (VKA) were used in conjunction with prostacyclin (33). Considering these data, increased use of prostacyclin in the REVEAL registry potentially provides enough anticoagulation or anti-platelet activity to mitigate any possible observed benefit of VKA therapy. Moreover, although the occurrence of fatal bleeding complications was similar in COMPERA and REVEAL, neither study was designed to capture all bleeding events. Increased bleeding risk in REVEAL (as a result of increased concurrent prostacyclin and VKA use) could theoretically increase morbidity and mortality, decreasing observed benefit of anticoagulation use in iPAH.
Even more interesting is the disparity between warfarin use patterns in the two registries; a point which more than any raises concern of comparability. The more recently published REVEAL registry reported a mean time on warfarin of 11.8 months, with 3, 9 and 16.5 months for the 25th, 50th and 75th percentiles, respectively. The mean INR was reported at 1.9 in the iPAH participants. While COMPERA does not provide mean INR measurements, limiting our ability to assess quality of anticoagulation, the duration of use is notably longer. Fifty-six percent of patients used warfarin for the entire 36-month follow-up period, with 85% anti-coagulated for >50% of the time (>18 months). These results raise the possibility that in iPAH there is a critical duration of anticoagulation that provides benefit—the majority of patients in REVEAL may not have reached this crucial threshold and therefore have no improvement in survival. Although REVEAL included a time-interval time-varying covariate model, this statistical strategy only addressed ever versus recent use, not cumulative duration.
The concern of immortal time bias (ITB) was raised in both the COMPERA and REVEAL manuscripts and a contemporary review in Circulation (2,14,23). Fundamentally, the concept of ITB implies that patients included in the warfarin group must survive long enough to start therapy, potentially biasing towards increased survival in the warfarin cohort; that is to say, that patients included in the no warfarin cohort may die from another cause prior to having a chance to start warfarin therapy (2,34). Study design in the REVEAL registry decreases the probability of ITB compared to COMPERA and may explain the lack of mortality benefit, but the degree to which this bias alters the results is uncertain.
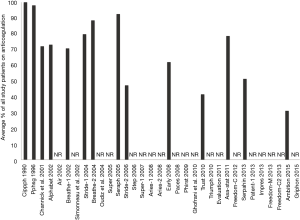
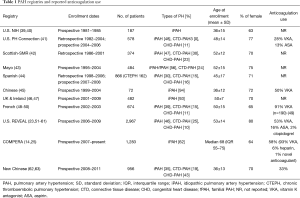
So where do we go from here? There are still only 10 studies specifically addressing anticoagulation in PAH and the data therein remains of questionable utility. The two most influential publications attempting to answer this question have conflicting results and debatably should not be compared in light of differing demographics. Moreover, a minor and decreasing proportion of randomized trials (Figure 1) and registries (Table 1) report the use of warfarin or other types of anticoagulation. COMPERA and REVEAL are the only 2 modern registries reporting anticoagulation use to provide direct analysis of morbidity and mortality related to this controversial therapeutic strategy. Almost more striking is the decline in reporting from randomized trials (Figure 1). In the first two decades of major PAH investigation, 50% reported the use of anticoagulation and the pooled percent of patients taking anticoagulants ranged from 47–100%. Since 2010, only approximately 30% of studies disclose anticoagulation, and use ranges from 31–79%. Whether this reduction in reporting reflects decreased anticoagulation use in practice or simply waning interest remains to be seen.
Full table
More robust data in the form of a well planned, international randomized controlled study would provide great insight into this topic (2). Alternatively, this area of clinical uncertainty would be well suited for a randomized registry study (64), an attractive, economic avenue that has not been successfully employed in PAH as of yet (65). While not without consequences (9,33), anticoagulation retains a place in treatment algorithms, prompting urgent reconciliation of this therapeutic dilemma in PAH.
Acknowledgements
None.
Footnote
Provenance: This is an invited Perspective commissioned by the Section Editor Yue Liu (Department of Cardiology, the First Affiliated Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hatton N, Ryan JJ. The complicated question of anticoagulation in pulmonary arterial hypertension: time to get some simple answers. Can J Cardiol 2014;30:850-2. [Crossref] [PubMed]
- Frantz RP. Whither Anticoagulation in Pulmonary Arterial Hypertension? Conflicting Evidence REVEALed. Circulation 2015;132:2360-2. [Crossref] [PubMed]
- Diehl P, Aleker M, Helbing T, et al. Increased platelet, leukocyte and endothelial microparticles predict enhanced coagulation and vascular inflammation in pulmonary hypertension. J Thromb Thrombolysis 2011;31:173-9. [Crossref] [PubMed]
- Hoeper MM, Sosada M, Fabel H. Plasma coagulation profiles in patients with severe primary pulmonary hypertension. Eur Respir J 1998;12:1446-9. [Crossref] [PubMed]
- Welsh CH, Hassell KL, Badesch DB, et al. Coagulation and fibrinolytic profiles in patients with severe pulmonary hypertension. Chest 1996;110:710-7. [Crossref] [PubMed]
- Fuster V, Steele PM, Edwards WD, et al. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 1984;70:580-7. [Crossref] [PubMed]
- White RJ. Thrombin and platelets in pulmonary hypertension?: a lot more than clot. Adv Pulm Hypertens 2012;11:19-24.
- Wagenvoort CA, Wagenvoort N. Primary pulmonary hypertension. Circulation 1970;42:1163-84. [Crossref] [PubMed]
- Roldan T, Landzberg MJ, Deicicchi DJ, et al. Anticoagulation in patients with pulmonary arterial hypertension: An update on current knowledge. J Heart Lung Transplant 2016;35:151-64. [Crossref] [PubMed]
- Caldeira D, Loureiro MJ, Costa J, et al. Oral Anticoagulation for Pulmonary Arterial Hypertension: Systematic Review and Meta-analysis. Can J Cardiol 2014;30:879-87. [Crossref] [PubMed]
- Ezedunukwe IR, Enuh H, Nfonoyim J, et al. Anticoagulation therapy versus placebo for pulmonary hypertension. Cochrane Database Syst Rev 2014;6:CD010695. [PubMed]
- Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 1992;327:76-81. [Crossref] [PubMed]
- Ngian GS, Stevens W, Prior D, et al. Predictors of mortality in connective tissue disease-associated pulmonary arterial hypertension: a cohort study. Arthritis Res Ther 2012;14:R213. [Crossref] [PubMed]
- Olsson KM, Delcroix M, Ghofrani HA, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the comparative, prospective registry of newly initiated therapies for pulmonary hypertension (COMPERA). Circulation 2014;129:57-65. [Crossref] [PubMed]
- Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol 2005;95:199-203. [Crossref] [PubMed]
- Frank H, Mlczoch J, Huber K, et al. The effect of anticoagulant therapy in primary and anorectic drug-induced pulmonary hypertension. Chest 1997;112:714-21. [Crossref] [PubMed]
- Ogata M, Ohe M, Shirato K, et al. Effects of a combination therapy of anticoagulant and vasodilator on the long-term prognosis of primary pulmonary hypertension. Jpn Circ J 1993;57:63-9. [Crossref] [PubMed]
- Storstein O, Efskind L, Müller C, et al. Primary pulmonary hypertension with emphasis on its etiology and treatment. Acta Med Scand 1966;179:197-212. [Crossref] [PubMed]
- Saeed W, Tiawari N, Sardar MR, et al. Effect of warfarin on long term pulmonary arterial hypertension (PAH) mortality: change of facts? Circulation 2011;124:A16034.
- Johnson SR, Mehta S, Granton JT. Anticoagulation in pulmonary arterial hypertension: a qualitative systematic review. Eur Respir J 2006;28:999-1004. [Crossref] [PubMed]
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. [Crossref] [PubMed]
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. Circulation 2009;119:2250-94. [Crossref] [PubMed]
- Preston IR, Roberts KE, Miller DP, et al. Effect of warfarin treatment on survival of patients with pulmonary arterial hypertension (PAH) in the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL). Circulation 2015;132:2403-11. [Crossref] [PubMed]
- Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012;141:354-62. [Crossref] [PubMed]
- Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 2013;168:871-80. [Crossref] [PubMed]
- Thenappan T, Ryan JJ, Archer SL. Evolving epidemiology of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;186:707-9. [Crossref] [PubMed]
- Olsson KM, Nickel NP, Tongers J, et al. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int J Cardiol 2013;167:2300-5. [Crossref] [PubMed]
- Huston J, Han FT, Ryan JJ. Another piece to the puzzle: linking the cardiac nervous system to atrial fibrillation in pulmonary arterial hypertension. Hypertension 2015;66:935-7. [Crossref] [PubMed]
- Ryan JJ, Butrous G, Maron BA. The heterogeneity of clinical practice patterns among an international cohort of pulmonary arterial hypertension experts. Pulm Circ 2014;4:441-51. [Crossref] [PubMed]
- Friedman R, Mears JG, Barst RJ. Continuous infusion of prostacyclin normalizes plasma markers of endothelial cell injury and platelet aggregation in primary pulmonary hypertension. Circulation 1997;96:2782-4. [Crossref] [PubMed]
- Yardumian DA, Machin SJ. Altered platelet function in patients on continuous infusions of epoprostenol. Lancet 1984;1:1357. [Crossref] [PubMed]
- Sakamaki F, Kyotani S, Nagaya N, et al. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation 2000;102:2720-5. [Crossref] [PubMed]
- Henkens IR, Hazenoot T, Boonstra A, et al. Major bleeding with vitamin K antagonist anticoagulants in pulmonary hypertension. Eur Respir J 2013;41:872-8. [Crossref] [PubMed]
- Ho AM, Dion PW, Ng CSH, et al. Understanding immortal time bias in observational cohort studies. Anaesthesia 2013;68:126-30. [Crossref] [PubMed]
- Channick R, Badesch DB, Tapson VF, et al. Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary hypertension: a placebo-controlled study. J Heart Lung Transplant 2001;20:262-263. [Crossref] [PubMed]
- Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of Treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2002;165:800-4. [Crossref] [PubMed]
- Oudiz RJ, Schilz RJ, Barst RJ, et al. Treprostinil, a prostacyclin analogue, in pulmonary arterial hypertension associated with connective tissue disease. Chest 2004;126:420-7. [Crossref] [PubMed]
- Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock AJ, Barst RJ, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med 2010;182:1171-7. [Crossref] [PubMed]
- D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343-9. [Crossref] [PubMed]
- Rich S, Dantzker D. Primary pulmonary hypertension: a national prospective study. Ann Intern Med 1987;107:216-23. [Crossref] [PubMed]
- Thenappan T, Shah SJ, Rich S, et al. Survival in pulmonary arterial hypertension: A reappraisal of the NIH risk stratification equation. Eur Respir J 2010;35:1079-87. [Crossref] [PubMed]
- Peacock AJ, Murphy NF, McMurray JJ, et al. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007;30:104-9. [Crossref] [PubMed]
- Kane GC, Maradit-Kremers H, Slusser JP, et al. Integration of clinical and hemodynamic parameters in the prediction of long-term survival in patients with pulmonary arterial hypertension. Chest 2011;139:1285-93. [Crossref] [PubMed]
- Escribano-Subias P, Blanco I, López-Meseguer M, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J 2012;40:596-603. [Crossref] [PubMed]
- Jing ZC, Xu XQ, Han ZY, et al. Registry and survival study in chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest 2007;132:373-9. [Crossref] [PubMed]
- Lee WT, Ling Y, Sheares KK, et al. Predicting survival in pulmonary arterial hypertension in the UK. Eur Respir J 2012;40:604-11. [Crossref] [PubMed]
- Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012;186:790-6. [Crossref] [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023-30. [Crossref] [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156-63. [Crossref] [PubMed]
- Humbert M, Sitbon O, Yaïci A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010;36:549-55. [Crossref] [PubMed]
- McGoon MD, Krichman A, Farber HW, et al. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc 2008;83:923-31. [Crossref] [PubMed]
- Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: Baseline characteristics from the REVEAL registry. Chest 2010;137:376-87. [Crossref] [PubMed]
- Barst RJ, McGoon MD, Elliott CG, et al. Survival in childhood pulmonary arterial hypertension: Insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012;125:113-22. [Crossref] [PubMed]
- Benza RL, Miller DP, Frost A, et al. Analysis of the lung allocation score estimation of risk of death in patients with pulmonary arterial hypertension using data from the REVEAL Registry. Transplantation 2010;90:298-305. [Crossref] [PubMed]
- Brown LM, Chen H, Halpern S, et al. Delay in recognition of pulmonary arterial hypertension: Factors identified from the REVEAL registry. Chest 2011;140:19-26. [Crossref] [PubMed]
- Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: Identifying systemic sclerosis as a unique phenotype. Chest 2010;138:1383-94. [Crossref] [PubMed]
- Frost AE, Badesch DB, Barst RJ, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: How REVEAL differs from historic and non-US contemporary registries. Chest 2011;139:128-37. [Crossref] [PubMed]
- Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: A report from the US-based REVEAL registry. Chest 2012;141:906-15. [Crossref] [PubMed]
- Shapiro S, Traiger GL, Turner M, et al. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest 2012;141:363-73. [Crossref] [PubMed]
- Farber HW, Miller DP, Poms AD, et al. Five-year outcomes of patients enrolled in the REVEAL registry. Chest 2015;148:1043-54. [Crossref] [PubMed]
- Farber HW, Foreman AJ, Miller DP, et al. REVEAL registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail 2011;17:56-64. [Crossref] [PubMed]
- Zhang R, Dai LZ, Xie WP, et al. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest 2011;140:301-9. [Crossref] [PubMed]
- Jiang X, Humbert M, Jing ZC. Idiopathic pulmonary arterial hypertension and its prognosis in the modern management era in developed and developing countries. In: Humbert M, Souza R, Simonneau G, editors. Pulmonary vascular disorders, progress in respiratory research. Basel: Karger; 2012:85-93.
- Lauer MS, D'Agostino RB Sr. The randomized registry trial--the next disruptive technology in clinical research? N Engl J Med 2013;369:1579-81. [Crossref] [PubMed]
- Ryan JJ, Rich JD, Maron BA. Building the case for novel clinical trials in pulmonary arterial hypertension. Circ Cardiovasc Qual Outcomes 2015;8:114-23. [Crossref] [PubMed]


