Tissue requirements in lung cancer diagnosis for tumor heterogeneity, mutational analysis and targeted therapies: initial experience with intra-operative Frozen Section Evaluation (FROSE) in bronchoscopic biopsies
Introduction
Transbronchial biopsy was first performed in 1963 with rigid bronchoscopy and flexible forceps (1). Then in 1970, the flexible fiberoptic bronchoscope was first introduced by Dr. Shigeto Ikeda. Throughout the 1970s–1980s, instruments were adapted to the flexible bronchoscope for biopsy through its working channel. The primary tools developed were the flexible forceps, needles, brushes, and curettes, all with many variations of sizes and different modifications. As flexible bronchoscopy could be performed as an outpatient procedure and did not require the resources previously needed for rigid bronchoscopy, pulmonologists quickly adopted flexible bronchoscopy as a primary tool for diagnosing lung cancer. Over time, technological advances improved imaging quality of the bronchoscope while often reducing its outer diameter while increasing the size of the working channel. Radiographs, fluoroscopy, CT, CT fluoroscopy, ultrasound, and more recently navigational bronchoscopy have all increased the ability to reach the peripheral lung lesions with improved diagnostic yield.
Despite all of these technologic advances and tools, we still are limited in our ability to consistently get diagnostic yields above 90% even in the most experienced operators’ hands. To add to the challenge, we have moved into a new phase in lung cancer treatment that relies heavily on specific mutation analysis of the individual’s lung cancer to apply targeted therapies. In the recent past, we only needed enough tissue to distinguish non small cell lung cancer (NSCLC) as the treatment was the same for all types of NSCLC. Now, there is the need for enough specimen to identify lung adenocarcinoma versus squamous cell carcinoma and then have enough to send for mutation analysis at a minimum. Tissue samples or cell block are required for this analysis. There is little in the literature to guide the bronchoscopist in approaching biopsy to ensure adequate specimens for this new requirement for lung cancer diagnostic testing and what will almost certainly be the need for more and different types of testing in the future. In 2013, results of electromagnetic navigation bronchoscopy (ENB) in combination with multiple biopsy instruments were published demonstrating the ability of forceps, 21 gauge SuperTrax needle, 22 gauge Wang needle, brush and washing to obtain a diagnosis with histologic and molecular characterization in lung cancer (2). Using ENB, each biopsy method was individually assessed for yield in lung adenocarcinoma showing a range of 71–90% (excluding washings which had a 27% yield). The Wang 22 gauge needle had the highest yield but the forceps had the highest percentage of cases being the only diagnostic specimen in the case. While these yields are good, they still represent up to a 30% failure rate in obtaining adequate tissue for just a diagnosis even at a top center in expert hands let alone the need for enough tissue to perform appropriate analytics. These results highlight the challenge for obtaining the histologic and molecular samples needed for the new paradigm in lung cancer treatments.
There are several critical elements to achieving a diagnosis of lung cancer bronchoscopically. Critical factors for bronchoscopic biopsy success include lesion characteristics, biopsy instrument, navigation accuracy, and specimen collection and analysis. Currently, the important characteristics that help define successful tissue acquisition are the proximity and relationship of the lesion to the airway, the size of the nodule/mass, type of tissue (benign versus malignant etc.), and its location. Advances in navigation increase the ability to get close to the lesion, but if the target lesion is outside the airway wall, a tool that can penetrate the wall must be selected for biopsy (3). Size of the lesion is important as larger lesions are more likely to have multiple airways leading to them and are a larger target to find. One challenge with some large lesions is necrosis. Significant necrosis decreases the quantity of viable cells for histologic and molecular analysis. Real time assessment of sample quality is often used to help guide biopsy location and biopsy tool selection. One technique used to assess the quantity and quality of the biopsy specimen for lung cancer molecular analysis is rapid on-site evaluation (ROSE) (4). Recently in a randomized controlled trial ROSE has been shown to prevent the need for rebiopsy to obtain more specimen for molecular profiling of the tumor in 1 out of 10 patients with advanced lung cancer undergoing endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) (5). Unfortunately, many institutions do not have access to a ROSE program for many reasons including but not limited to the substantive cost associated with it given that it requires a cytotechnologist to be present at the bronchoscopy to process and evaluate the samples. However, most institutions with an operating room have a pathology service that will process and review intraoperatively specimens from surgical cases. We report our experience at a community hospital setting using intra-procedural Frozen Section Evaluation (FROSE) of bronchoscopic biopsy specimens (combination of biopsy tools including forceps and needle) of suspected lung cancer and our ability to successfully diagnose and obtain molecular profiling of the tumor.
Our decision to use frozen section as an intraoperative adequacy assessment technique was based on several factors. These were: (I) the availability of pathologists and/or cytotechnologists to be present for intraoperative cytology preparation and interpretation, in the context of the reasonable frequency of interventional pulmonology procedures at our institution given our resources; (II) the resources already in place for frozen section processing and interpretation; (III) preference of our pathologists for frozen section histologic interpretation versus cytological assessment; (IV) related to the preceding reason, avoidance of some of the ambiguities inherent in the assessment of “adequacy” versus “diagnostic” in the setting of intraoperative cytology assessment. In our experience, there is lingering confusion and potential for miscommunication between pathologists, cytotechnologists, and clinicians about what these terms imply. In particular, our pulmonologists wanted to avoid situations where “adequate” intraoperative assessments returned “atypical” final diagnoses, versus unambiguous diagnoses of “benign” or “malignant”. While these ambiguities also hold to a certain extent in the context of frozen section interpretation, there is a higher level of comfort and confidence among pathologists at our institution, and perhaps generally, in assessing frozen sections where the at times confusing and confounding concern of “adequacy”, often felt in the cytological context, is less of a factor in interpretation; (V) frozen section can give some sense of the quantity of tissue available for potential ancillary studies. As cited above, while the use of ROSE does appear to improve the quality of cell block material for additional studies, it seemed to us that the amount of tumor present in the frozen section was something directly and easily perceivable and predictive of the amount of tumor present in the given sample or site of biopsy, allowing for useful direction as to whether more tissue was or was not needed which has substantive utility given the changing nature of the landscape, ‘druggable targets’, etc.; (VI) finally, and perhaps most importantly, we are physically able to freeze these samples like other more routine frozen specimens; that is to say, the method of tissue procurement employed by our interventional pulmonologists does not necessitate cytological interpretation. We are routinely given several square millimeters of sample which can easily be divided and frozen according to our judgment and the needs of the case.
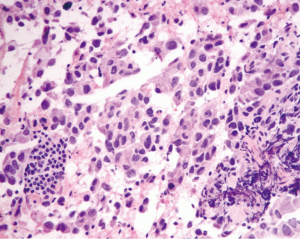
We have used intraoperative frozen section for transbronchial and mediastinal lymph node biopsies (Figure 1) for over three years, dating back to 2011–2012 when the need for stricter tissue management became apparent. The following discussion looks at some of the performance characteristics of this technique over a recent six month period.
Methods
A retrospective analysis of all interventional pulmonology cases with intraoperative frozen section performed between February 1st, 2015 and July 31st, 2015 at MedStar Franklin Square Medical Center in Baltimore, MD, was performed. The following data points were gathered from each individual case:
- Site of frozen section;
- Frozen section diagnostic category (benign vs. malignant vs. atypical) and specific frozen section diagnosis (e.g., adenocarcinoma);
- Total sites sampled;
- Permanent section diagnostic category of frozen section sample (benign vs. malignant vs. atypical);
- Whether immunohistochemical studies were performed;
- Overall final diagnosis of the case including non-frozen samples;
- Results of molecular studies, if ordered;
- Results analyzed to determine what percentage of the time does frozen section return a definite diagnosis of benign or malignant versus a diagnosis of “atypical”, and what is the concordance of the frozen section diagnosis with the ultimate permanent section diagnosis and in those cases where molecular studies were ultimately required, what percentage of those cases was adequate for all ordered testing.
Results
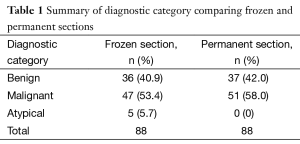
A total of eighty-eight interventional pulmonology cases employing a frozen section in at least one site were identified between February 1st and July 31st 2015. Table 1 shows the number of cases with frozen section diagnoses of benign, malignant, or atypical and the number of benign and malignant permanent section diagnoses made on these same cases.
Full table
In 5 of the 88 cases (5.7%), a diagnosis of atypia was made. In the remainder of the cases (94.3%) a definite frozen section diagnosis of benign or malignant was made. The benign diagnoses most often consisted of benign lymph node tissue or non-caseating granulomata. The malignant diagnoses most often consisted of non-small cell carcinoma, with a subset of cases reporting more specific frozen section diagnoses, including adenocarcinoma, squamous cell carcinoma, or small cell carcinoma. Of the five atypical diagnoses, two were atypical lymphoid populations, one of which was diagnosed as mantle cell lymphoma with supporting flow cytometry and the other as benign lymphoid tissue, also with supporting flow cytometry. The other three atypical diagnoses were notable either for extensive necrosis or extreme scantiness of sample. In all three of these cases, an ultimate diagnosis of malignancy was made on permanent section of the frozen material. The concordance of frozen section diagnoses of benign and malignant were 100% concordant with the ultimate permanent section diagnoses of the frozen material. It should be noted that this does not mean that additional sites sampled in the same case that were not frozen necessarily showed the same thing as the frozen section material. For example, a common situation is one in which a mediastinal lymph node is sent for frozen section and is benign, and is then followed by a transbronchial biopsy of a lung lesion sent for permanent section only which ultimately demonstrates malignancy.
We then analyzed the subset of cases that met criteria for molecular studies, and of that number how many cases contained adequate tissue for testing (Table 2) shows results relevant to this question.

Full table
Cases were sent for ancillary studies if they were high stage as determined by the interventional procedure itself (e.g., positive mediastinal lymph nodes), or by provided clinical information. The ancillary studies most often consisted of molecular tests for EGFR and KRAS mutations and fluorescence in-situ hybridization for ALK and ROS1, though in a subset of cases only tests for EGFR and ALK were ordered. Thirteen of the eighty-eight cases (14.8%) were ultimately sent for molecular analysis. Of these, twelve of thirteen (92.3%) cases were adequate to perform all ordered molecular testing. In the one case where there was a failure (ROS1), not all tissue that could have been sent was sent. In all cases there was sufficient tissue to perform EGFR and ALK testing.
Discussion
The difficulties inherent in the procurement and judicious management of small biopsies and FNAs of non-small cell lung carcinoma are not unlike many others in pathology and medicine generally. Always the challenge is to do “more with less”. We feel that the use of intraoperative frozen section for transbronchial and mediastinal lymph node core needle biopsies is an effective tool in meeting that challenge. Furthermore, we believe that this technique could be successfully employed at other institutions with a view toward enhanced communication between pathologist and pulmonologist and efficient division of tissue for diagnostic and ancillary testing needs.
With the emerging importance of molecular diagnostics to guide therapy, a multidisciplinary approach is needed to set a consistent strategy for obtaining and preserving tissue samples optimized to perform studies such as DNA sequence analysis, fluorescence in situ hybridization (FISH), and RNA-based studies. The challenge of successfully developing guidelines with such wide variation in infrastructure and expertise from one institution to another was recognized by the top international professional societies in the latest IASLC/ATS/ERS International Multidisciplinary Classification of Lung Cancer published in 2011 (6). Identifying best practices that have been successful in different medical environments may offer a menu of customized approaches to optimizing diagnostic lung cancer services at an individual institution. As our ability to image, utilize computer software and electromagnetic devices to navigate to small areas of the lung with precision continue to mature, the final and most crucial element in the biopsy of these lesions is intraprocedural feedback to the operator on the quality and content of the biopsy. Historically this has been performed for surgeons in the operating room on larger tissue samples, but due to the small size of transbronchial forceps biopsies or even transbronchial needle aspiration (TBNA) samples, frozen section has not been a routine technique for bronchoscopy. As we discussed earlier, ROSE has become the predominant technique used to provide intraprocedural feedback for bronchoscopic biopsies in the diagnosis of the pulmonary nodule, it is not available at all centers and because it is a cytological evaluation, it cannot give detailed feedback on thicker tissue specimens that we obtain on transbronchial forceps biopsies. Understanding the type and quality of the specimens will be extremely important in consistently obtaining tissue and maintaining the highest possible level of diagnostic accuracy and preserving adequate tissue for ancillary studies, not only those presently well-established (EGFR, ALK) but also any and all possible molecular markers that may become important in the future.
Our approach to the sampling and tissue management of non-small cell carcinoma has developed through a close partnership between our pathologists and interventional pulmonologists. Intraoperative adequacy assessment is a well-established practice in the setting of fine needle aspiration. ROSE is a cytological technique that has been shown to decrease the number of lesions sampled and number of transbronchial needle aspirations performed, as well improved the quality of cellblock allowing for potential benefit of immunohistochemistry and molecular pathology (7). In our institution, we follow several of the suggestions given by Travis et al. (8) for the management of small biopsies of non-small cell carcinoma, including a limited approach to immunohistochemical staining (typically one marker each for adenocarcinoma and squamous cell carcinoma) and frequent splitting of the sample into multiple paraffin blocks. As at many institutions, ROSE is not available. In an attempt to increase our diagnostic capabilities and ability to provide adequate samples for molecular analysis in lung cancer cases, we began to use the FROSE technique for bronchoscopic specimens. Similar to ROSE, intraoperative adequacy assessment is provided to the bronchoscopist by phone directly from the pathology lab after immediate review. In addition to cytology samples used with ROSE, we also gave intraprocedural feedback on tissue samples evaluated with FROSE. This approach was customized to our institution’s resources and was highly effective in obtaining adequate specimens for molecular marker analysis in cases of lung cancer.
Further investigation is required to determine if the addition of tissue evaluation and qualitative feedback to the bronchoscopist that can be provided with FROSE is useful when compared with ROSE (cytological evaluation only) or no analysis of the specimen during the procedure. While there is communication between the pathologist and interventional pulmonologist as to how much tissue is present in submitted samples, at our institution, has been generally qualitative in nature, not only in terms of the amount of tissue present in the frozen section (“scant” versus “extensive”) but also in terms of the pulmonologist response to this information. This interaction between the pathologist and the bronchoscopist and the effect on the procedure may be very critical in the biopsy results. Changes in the amount of sites sampled and/or biopsies taken in situations where the frozen tissue is reported to be scant or extensive, as well as to generate quantitative data as to the amount of tumor actually present in these various situations in terms of millimeters squared or cell counts could positively or negatively affect the outcome, procedure length and complications. Our series is limited by its retrospective evaluation and possible selection bias for cases where FROSE was utilized. Further study is needed, but in our institution FROSE for bronchoscopic biopsies appears to be a viable and in many respects an advantageous technique for obtaining molecular markers in lung cancer patients.
Conclusions
In medical centers where ROSE may not be available, the use of FROSE by the local pathologist can be an effective technique to obtain adequate tissue and cytological samples for the diagnosis and molecular profiling of lung cancers. Further prospective study in bronchoscopic tissue sampling techniques to obtain the optimum quantity and quality of samples for molecular profiling of lung cancers for targeted treatments is needed.
Acknowledgements
None.
Footnote
Conflicts of Interest: Subsequent to the completion of the supplement, Dr. Krimsky is a part-time employee of Medtronic. Prior to that he was a consultant for Medtronic with intellectual property rights. The other authors have no conflicts of interest to declare.
Disclaimer: The views expressed in this lecture are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. The identification of specific products or scientific instrumentation does not constitute endorsement or implied endorsement on the part of the author, DoD, or any component agency. While we generally excise references to products, companies, manufacturers, organizations, etc. in government produced works, the abstracts produced and other similarly situated researcher presents a special circumstance when such product inclusions become an integral part of the scientific endeavor.
Ethical Statement: The study protocol was approved by IRB # 2 Baltimore (Protocol No. 2016-164).
References
- Andersen HA, Fontana RS, Harrison EG Jr. Transbronchoscopic lung biopsy in diffuse pulmonary disease. Dis Chest 1965;48:187-92. [Crossref] [PubMed]
- Ha D, Choi H, Almeida FA, et al. Histologic and molecular characterization of lung cancer with tissue obtained by electromagnetic navigation bronchoscopy. J Bronchology Interv Pulmonol 2013;20:10-5. [Crossref] [PubMed]
- Mazzone P, Jain P, Arroliga AC, et al. Bronchoscopy and needle biopsy techniques for diagnosis and staging of lung cancer. Clin Chest Med 2002;23:137-58. ix. [Crossref] [PubMed]
- Bulman W, Saqi A, Powell CA. Acquisition and processing of endobronchial ultrasound-guided transbronchial needle aspiration specimens in the era of targeted lung cancer chemotherapy. Am J Respir Crit Care Med 2012;185:606-11. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With and Without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 2015;148:1430-7. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Wong RW, Thai A, Khor YH, et al. The Utility of Rapid On-Site Evaluation on Endobronchial Ultrasound Guided Transbronchial Needle Aspiration: Does It Make a Difference? Journal of Respiratory Medicine 2014;2014. doi: [Crossref]
- Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:668-84. [Crossref] [PubMed]