Recent clinical innovations in thoracic surgery in Hong Kong
Introduction
Substantial innovations in thoracic surgery have occurred over the past 20 years, allowing thoracic surgeons worldwide to perform less invasive and more personalised treatments for thoracic disorders. The popularity of major lung resection using single port video-assisted thoracic surgery (VATS) in Asia has posed challenges to surgeons, who must acquire new skills and knowledge especially for managing instruments placed unidirectionally and for intraoperative localisation of small pulmonary nodules when tactile feedback is limited. These issues have necessitated industry-clinician collaboration to develop ergonomic instruments preventing instrumental fencing during minimally invasive surgeries. With increasing knowledge and understanding of the importance of different lung tumour subtypes, the concept of individualised surgical therapy is on the horizon. A hurdle to realize this concept is the ability to rapidly and accurately identify tumour subtypes intraoperatively which can guide the appropriateness of lesser sublobar resections. On the other end, for large tumours requiring chest wall resection and reconstructions, several novel approaches have come in vogue that champions the ideal of personalized surgery. Herein, we will discuss the above topics and introduce several clinical innovations from Hong Kong.
Single-port thoracic surgery in Hong Kong
Single-port VATS has evolved rapidly since the first report on sympathectomy in 2002 (1). Such developments have led to a paradigm shift in the approach to major lung resection after a milestone, world-first report of single port VATS lobectomy in 2011 by Dr. Gonzalez’s group from Spain (2). The single port VATS approach is a promising, minimally invasive thoracic surgery method that is rapidly gaining popularity worldwide.
However, there remain some concerns with single-port VATS in Asian patients, mostly due to a high incidence of tuberculosis and stature-related complications in this particular population (3). Theoretically, a high prevalence of histological tuberculosis in Asian patients could complicate dissections of pleural adhesions and calcified lymph nodes. Nevertheless, Yim et al. demonstrated that histological findings of tuberculosis did not hamper VATS procedures in Asian patients (4). Comparatively smaller rib cages in Asian patients often pose another technical challenge with respect to instrument placement, as well as vascular stapling during single port VATS.
To further elucidate the effectiveness of single-port VATS in Asian patients, we recently reported a study of 150 consecutive cases treated with single-port VATS major lung resection between 2012 and 2014 (5). No 30-day mortality was found, whereas two cases died within 60 days postoperatively from surgically unrelated causes. In all, single port VATS was successfully performed in 142 cases, yielding a conversion rate of 5.3%, which is slightly higher than the series report from Gonzalez-Rivas’s group (5/102, 2.9%) (6). However, more patients in our cohort that required conversion underwent two-port VATS (n=6) rather than an open thoracotomy (n=2) compared with the Spanish experience. Reasons for conversion included bleeding, difficult lymph nodes, excessive adhesion, and tumours that crossed fissures. In addition, the operation time, number of lymph nodes dissected, and complication rate were comparable to those of a European population (6), indicating that single-port VATS is safe in the Asian population.
Narrower rib spaces can also cause difficulty when placing the 10-mm thoracoscope, angulated clamp or grapser, and endostapler together through a single intercostal space. Double-hinged VATS-specific instruments with a narrow shaft help surgeons to reach further into the chest cavity. Greater lens flexibility, as with the wide-angled rigid 120° thoracoscope (EndoCAMeleon, Karl Storz, Germany), may improve visibility particularly for surgeons still mastering the technique and in difficult cases (7). Furthermore, it is possible that a 5-mm thoracoscope would avoid instrumental torqueing to some extent while providing unambiguous images. In addition, we also used a porcine model to show that the novel four-row endocutter (Echelon FlexTM, Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA) with a narrower anvil can achieve haemostasis equivalent to that of a standard six-row stapler (8). The thinner shaft with a wider range of articulation facilitates passing of critical vascular structures during single-port access, especially when dealing with fragile pulmonary vessels (Figure 1). In addition, such a device may be favoured by Asian surgeons when limited dissection of the vessels is required, for example when performing a single port VATS segmentectomy for early-stage pulmonary lesions.
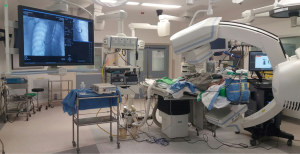
Hybrid operation room (OR) for thoracic surgery
Real-time hookwire implantation to localise pulmonary lesions
In the era of minimally invasive thoracic surgery, diagnosis and intraoperative localisation of pulmonary nodules can be challenging, especially in single-port VATS with limited access. Manual palpation is particularly difficult through a single incision, and may be more problematic with part-solid lesions with a high proportion of ground-glass opacity (GGO). There are many classic preoperative techniques, most of which are computed tomography (CT)-guided, and often use dye, contrast medium, radionucleotide labelling, or hookwire/microcoil implantation to help localise small lesions intraoperatively. Nevertheless, such techniques require the localisation to be performed in the radiology suite followed by surgery in the OR. As a result, patients might experience discomfort and suffer from various complications such as pneumothorax or dislodgement of the localising material (9).
The idea of integrating real-time on-table image guidance with a hybrid OR into thoracic surgery, namely image-guided VATS, was first reported by Brigham and Women’s Hospital in 2013. In their initial study, 23 patients with small pulmonary lesions received conventional thoracoscopic surgery after T-shaped fiducial placement under intraoperative C-arm CT (10). We subsequently reported the world-first image-guided single-port VATS (iSPVATS) procedure of a lady who had a GGO nodule that underwent Dyna-CT-guided hookwire placement in a hybrid OR followed by intraoperative frozen section and immediate single port VATS lobectomy (11). The amount of time needed for the localization procedure was 30 min; the radiation exposure level was acceptable as limited number of scans were performed, similar to the conventional percutaneous technique in radiology suite. More importantly, on table real-time Dyna-CT can provide important information when sublobar resection is considered. Pulmonary deflation after beginning one-lung ventilation may theoretically cause wire dislodgement, but this could also be solved immediately by re-localising the lesion in the hybrid unit.
Notably, our hookwire iSPVATS technique would work perfectly for peripheral lesions even of subcentimeter size, and may reduce the risk of complications associated with implantation. However, a multidisciplinary team of surgeons, radiologists, and anaesthetists must be fully trained. Additionally, if multiple lesions require localisation, other methods such as intraoperative ultrasonography may be more appropriate (12).
Electromagnetic navigation bronchoscopy (ENB) with real-time adjustment
ENB technology has developed over the last decade into a useful diagnostic tool to detect peripheral lung lesions (13). Localisation of the nodules can be up to 93% accurate when combining ENB with endobronchial ultrasound (14). Currently, we are running a prospective trial to investigate the accuracy, diagnostic effectiveness, and safety of ENB superDimension (superDimension, Inc., Plymouth, MN, USA) in the Asian population. However, identification of subcentimetre lesions with a high GGO content using ENB can be problematic due to its navigational error of 4 to 6 mm, as well as reduced imaging capabilities from radial endobronchial ultrasound and standard fluoroscopy. To further increase the accuracy of ENB, we recently reported the world-first use of unparalleled real-time images from Dyna-CT to guide ENB biopsy in the hybrid OR (15) (Figure 2). An 8-mm lesion in the right middle lobe was successfully localised and diagnosed through ENB. Normally, we perform two intraoperative scans: the first to identify any minor misdirection and the second to confirm intralesional placement of the biopsy needle. In particular, Dyna-CT guides surgeons with respect to the direction of the catheter tip for needle aspiration, thus greatly improving its accuracy by avoiding potential navigational error, especially when dealing with lesions less than 10 mm.
Further development of this technique may be advanced by using dye labelling (16) or fiducial placement (17) of the lesion through the ENB route, potentially serving as adjunct technology for localisation, or even locoregional ablation for small peripheral lung nodules; undoubtedly, the hybrid OR allows such techniques to be conducted more precisely.
Technological innovations in surgical devices
The classic rigid design of the scope with a 30 degree lens is an advance of paramount importance that facilitates exploration of the chest cavity under VATS. However, surgeons must direct the stiff shaft correctly to expand the field of view (FOV), which can unfortunately cause fencing or colliding of the instruments in single-port VATS. The technique of having an integrated surgical instrument unit combining various components (e.g., an endoscope, a retraction device, and an energy source) to minimize undesirable instrument-instrument interaction has showed encouraging results in minor procedures. We reported the world-first successful use of a modern single-instrument unit VasoView (Maquet Inc., Rastatt, Germany) Hemopro device for single-port VATS bilateral sympathectomy. Through a 1-cm incision, this instrument, originally designed for endoscopic vein harvesting, can be placed into the thoracic cavity, and carbon dioxide insufflation is achieved via the intrinsic instrument channel, allowing satisfactory sympathectomy (18). Nevertheless, the relatively simple integrated instrument device, such as VasoView, would not be sufficient for more complex surgeries, for example performing major lung resections.
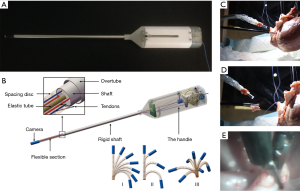
A flexible endoscope (Endo-EyeTM, Olympus, Tokyo, Japan) with a bronchoscopy-like motion has been reported to facilitate single-port VATS (19). However, the curved tip design of the Endo-Eye, without proper positioning, can occasionally occupy more space than a straight scope, thereby causing interference among instruments. To reduce instrumental collision without sacrificing the FOV, we designed a cardioscope prototype that has an telescopically adjustable, flexible distal tip (20). As a result, it may be more dexterous with a wider FOV compared to that of a conventional rigid scope, but also has the advantage of adjustable flexible tip length that can avoid instrument interference at the operating site (Figure 3). The challenge of improving the visual resolution of the cardioscope is currently being investigated which would further increase its clinical applicability.
Another idea, perhaps more inspiring, is to finally eliminate the need for an assistant-held thoracoscope through the surgical access point (21). Several mobile cameras could be inserted into the pleural cavity via one incision and held against the chest wall by magnets, replacing the conventional thoracoscope. Wireless cameras with a durable battery and own light source could transmit images to a receiver adjoining the monitor directly. Moreover, a circumferential FOV could be obtained by incorporating the views from the different cameras. By hanging the wireless cameras on an imitation chest wall modelled on human dimensions, we found the prototype of this novel system performed well in terms of sliding, rotating, and provision of multiple viewing angles (22) (Figure 4). Interestingly, the wireless steerable camera might be more suitable for VATS by providing superior stability against the rigid chest wall, compared with the initial design for the abdomen (21).

Intraoperative subtype identification for adenocarcinoma
Sublobar resection is often preferred over lobectomy to treat peripheral small pulmonary lesions detected on CT scans for sparing lung tissue and function. A recent retrospective study indicated that, for adenocarcinomas under 2 cm, segmentectomy can achieve oncologically equivalent outcomes to lobectomy (23). However, different subtypes may affect prognosis following resection, and to complicate matters further, lung adenocarcinomas that mostly present with part-solid attenuation on CT are thought to consist of heterogeneous subtypes, which also influence prognosis (24). A pulmonary nodule with a low consolidation proportion is generally considered radiologically non-invasive; differentials include an adenocarcinoma in situ, a minimally invasive adenocarcinoma, or a lepidic predominant adenocarcinoma, all of which can be treated with sublobar resection with excellent survival rates (25).
Notably, our recent study demonstrated that stage I adenocarcinoma with non-predominant micropapillary or solid growth components yields a significantly worse relapse-free survival and thus may not be suitable for sublobar resection (24). In fact, nearly 50% of adenocarcinomas had a micropapillary or solid component greater than 5% of the entire tumour (26), which is considered to indicate early recurrence after sublobar resection (27). Although intraoperative frozen section showed promising results in differentiating invasive adenocarcinoma from pre-invasive lesions (28), the sensitivity of detecting micropapillary or solid patterns remains disappointing (13.3–37% for micropapillary and 50–69% for solid patterns) (26,29). Sampling issues and interpretation errors are the major problems. Therefore, optimal intraoperative diagnosis of such components is still problematic.
Development of a rapid and accurate method intraoperatively to identify the predominant subtype of small adenocarcinoma lesion, which may be in the form of a molecular assay, would precisely guide surgical therapy. So far, the precise proteomic differences among the various invasive and less-invasive forms of lung adenocarcinoma using high throughput techniques such as antibody array have not been properly explored. Hopefully, comparison of the proteomic profiles of different subtypes will help clinicians to develop an intraoperative method to identify the micropapillary/solid pattern [e.g., using the semi-dry dot-blot method based on different antigen-antibody reactions (30)], allowing surgeons to personalise the extent of resection accordingly. Such important research development is on-going at Chinese University together with our Mainland China partners.
Chest wall reconstruction
Surgical repair of large thoracic wall defects resulting from the resection of sizable lesions is challenging. Sufficient reconstructions of the chest wall using various materials with or without soft tissue or patch coverage improves the quality of life and reduces functional impairment after extensive resection. A methylmethacrylate cement sandwich is widely used for repair (31,32), but uncommon complications such as toxicity, prosthesis infection, poor anchorage and fracture of the material, with associated chronic pain, may occur. Titanium materials, with their high strength-to-weight ratio and superior biological compatibility, are increasingly preferred for rigid structural reconstruction. Though the benefits of titanium devices over the older techniques remain unproven, it is possible that restoring rib continuity may preserve ventilation and be more tolerable by avoiding large cement prostheses (33).
Two main types of titanium device, i.e., clips and bars (STRATOSTM; MedXpert GmbH, Heitersheim, Germany) and plates and screws (MatrixRib; Synthes Inc., West Chester, PA, USA), are commercially available. In detail, the titanium clips and bars have a higher titanium component, which allows pliable securing of the ribs but can be more prone to fracture (34). In contrast, titanium plates that use screws for security are generally stronger due to use of titanium alloy with aluminium and niobium. We recently reported our technique for using MatrixRib system for chest wall reconstruction and described the essential steps and potential pitfalls of this technology (35). In particular, the plates should be placed parallel to the rib contour, and surgeons must be sure to place the drill, plates and screws over the thickest aspect of the rib to fit the measured screw length. Additionally, the mesh covering the inner layer should be approximately 2 cm larger than the defect circumferentially, to avoid lung herniation and detachment. Although the ergonomic contour of the MatrixRib plate with locking screws would prevent areas of high focused stress, fractures can very rarely still occur, due to excessive repeated or external stress (36). Therefore, patients should be made aware of this risk.
In the future, real-time image-guided planning in the hybrid theatre could better tailor the implant to each individual. Perhaps more excitingly, thanks to the development of three-dimensional printing using bioabsorbable materials (37), or even bioscaffolds incorporating patients’ own cells, surgeons may immediately transfer images from the hybrid theatre Dyna-CT into bioengineered replacement prostheses, leading to more highly customised reconstructions (32) (Figure 5). The challenges of significantly improving the 3D printing speed, sterilization of implant, to mention only a few, are yet to be overcome.

Conclusions
Single-port VATS major lung resection in Asian patients has had comparable outcomes to conventional approaches, and this technique is constantly evolving. Technological innovations such as the hybrid OR with Dyna-CT capabilities and more may enhance operating efficiency and safety, especially by allowing localisation, marking and biopsy of subcentimetre lesions via single port VATS. The unidirectional placement of the thoracoscope with surgical instruments may result in a limited range and angle of vision, which remains a major problem in thoracoscopic surgeries. Recent innovations in thoracoscope technology, such as the wide variable angled lens or flexible thoracoscope, have helped to lessen these shortcomings. Perhaps more encouragingly, the wireless steerable endoscope systems can improve the surgical FOV without occupying incisional space, thus eliminating fencing between the endoscope and instruments.
The revival of sublobar resection for early-stage lung adenocarcinoma carries serious concerns in identifying certain growth components such as micropapillary or solid patterned lesions, pre- or intra-operatively; further proteomic research may help to design an on-table diagnostic kit to account for this drawback. In addition, chest wall reconstructions may follow suit by becoming more individualised and precise, thanks to enhancements of surgical materials, 3D printing and advance real-time imaging. The thoracic surgical community in Hong Kong has taken up some of these challenges and produced clinical innovations and new ideas that will hopefully improve outcomes for our patients in the future.
Acknowledgements
Funding: This work was supported by Research Grants Council (RGC) General Research Fund (GRF) [14117715].
Footnote
Conflict of Interest: ZR Zhao, DR Situ and Z Li have no conflicts of interest to declare. CS Ng has an electromagnetic navigational bronchoscopy system SuperDimension Version 7 on loan from Medtronic. CS Ng is a member of the Asia-Pacific Thoracic Advisory Board, Ethicon, Johnson & Johnson, US.
References
- Nesher N, Galili R, Sharony R, et al. Videothorascopic sympathectomy (VATS) for palmar hyperhidriosis:summary of a clinical trial and surgical results. Harefuah 2000;138:913-6, 1008. [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Ng CS. Uniportal VATS in Asia. J Thorac Dis 2013;5 Suppl 3:S221-5. [PubMed]
- Yim AP, Izzat MB, Lee TW. Thoracoscopic surgery for pulmonary tuberculosis. World J Surg 1999;23:1114-7. [Crossref] [PubMed]
- Ng CS, Kim HK, Wong RH, et al. Single-Port Video-Assisted Thoracoscopic Major Lung Resections: Experience with 150 Consecutive Cases. Thorac Cardiovasc Surg 2016;64:348-53. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Ng CS, Wong RH, Lau RW, et al. Single port video-assisted thoracic surgery: advancing scope technology. Eur J Cardiothorac Surg 2015;47:751. [Crossref] [PubMed]
- Ng CS, Pickens A, Siegel JM, et al. A novel narrow profile articulating powered vascular stapler provides superior access and haemostasis equivalent to conventional devices†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i73-i78. [PubMed]
- Zhao ZR, Lau RW, Ng CS. Hybrid theatre and alternative localization techniques in conventional and single-port video-assisted thoracoscopic surgery. J Thorac Dis 2016;8:S319-27. [PubMed]
- Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS) - phase I-II clinical trial. J Surg Oncol 2015;112:18-25. [Crossref] [PubMed]
- Ng CS, Man Chu C, Kwok MW, et al. Hybrid DynaCT scan-guided localization single-port lobectomy. Chest 2015;147:e76-8. [corrected]. [Crossref] [PubMed]
- Rocco G, Cicalese M, La Manna C, et al. Ultrasonographic identification of peripheral pulmonary nodules through uniportal video-assisted thoracic surgery. Ann Thorac Surg 2011;92:1099-101. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest 2007;131:1800-5. [Crossref] [PubMed]
- Chee A, Stather DR, Maceachern P, et al. Diagnostic utility of peripheral endobronchial ultrasound with electromagnetic navigation bronchoscopy in peripheral lung nodules. Respirology 2013;18:784-9. [Crossref] [PubMed]
- Ng CS, Yu SC, Lau RW, et al. Hybrid DynaCT-guided electromagnetic navigational bronchoscopic biopsy†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i87-i88. [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5; discussion 475-6. [Crossref] [PubMed]
- Anantham D, Feller-Kopman D, Shanmugham LN, et al. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility study. Chest 2007;132:930-5. [Crossref] [PubMed]
- Ng CS, Lau RW, Wong RH, et al. Single-port vasoview sympathectomy for palmar hyperhidrosis: a clinical update. J Laparoendosc Adv Surg Tech A 2014;24:32-4. [Crossref] [PubMed]
- Yang Y, Bao F, He Z, et al. Single-port video-assisted thoracoscopic right upper lobectomy using a flexible videoscope. Eur J Cardiothorac Surg 2014;46:496-7. [Crossref] [PubMed]
- Li Z, Oo MZ, Nalam V, et al. Design of a Novel Flexible Endoscope - Cardioscope. ASME 2015 International Design Engineering Technical Conferences and Computer & Information in Engineering Conference (IDETC/CIE 2015) at Boston, Massachusetts.
- Cadeddu J, Fernandez R, Desai M, et al. Novel magnetically guided intra-abdominal camera to facilitate laparoendoscopic single-site surgery: initial human experience. Surg Endosc 2009;23:1894-9. [Crossref] [PubMed]
- Li Z, Ng CS. Future of uniportal videoassisted thoracoscopic surgery—emerging technology. Ann Cardiothorac Surg 2016;5:127-32. [Crossref] [PubMed]
- Veluswamy RR, Ezer N, Mhango G, et al. Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: Impact of Histology. J Clin Oncol 2015;33:3447-53. [Crossref] [PubMed]
- Zhao ZR, Xi SY, Li W, et al. Prognostic impact of pattern-based grading system by the new IASLC/ATS/ERS classification in Asian patients with stage I lung adenocarcinoma. Lung Cancer 2015;90:604-9. [Crossref] [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ≤ 3 cm: accuracy and interobserver agreement. Histopathology 2015;66:922-38. [Crossref] [PubMed]
- Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. [Crossref] [PubMed]
- Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. [Crossref] [PubMed]
- Trejo Bittar HE, Incharoen P, Althouse AD, et al. Accuracy of the IASLC/ATS/ERS histological subtyping of stage I lung adenocarcinoma on intraoperative frozen sections. Mod Pathol 2015;28:1058-63. [Crossref] [PubMed]
- Tomoshige K, Tsuchiya T, Otsubo R, et al. Intraoperative diagnosis of lymph node metastasis in non-small-cell lung cancer by a semi-dry dot-blot method†. Eur J Cardiothorac Surg 2016;49:617-22. [Crossref] [PubMed]
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5; discussion 1725-6.
- Ng CS. Recent and Future Developments in Chest Wall Reconstruction. Semin Thorac Cardiovasc Surg 2015;27:234-9. [Crossref] [PubMed]
- Billè A, Okiror L, Karenovics W, et al. Experience with titanium devices for rib fixation and coverage of chest wall defects. Interact Cardiovasc Thorac Surg 2012;15:588-95. [Crossref] [PubMed]
- Stefani A, Nesci J, Morandi U. STRATOS™ system for the repair of pectus excavatum. Interact Cardiovasc Thorac Surg 2013;17:1056-8. [Crossref] [PubMed]
- Ng CS, Ho AM, Lau RW, et al. Chest wall reconstruction with MatrixRib system: avoiding pitfalls. Interact Cardiovasc Thorac Surg 2014;18:402-3. [Crossref] [PubMed]
- Ng CS, Wong RH, Kwok MW, et al. Delayed fracture of MatrixRIB precontoured plate system. Interact Cardiovasc Thorac Surg 2014;19:512-4. [Crossref] [PubMed]
- Hwang TJ, Kiang C, Paul M. Surgical applications of 3-dimensional printing and precision medicine. JAMA Otolaryngol Head Neck Surg 2015;141:305-6. [Crossref] [PubMed]


