Localized malignant pleural sarcomatoid mesothelioma misdiagnosed as benign localized fibrous tumor
Introduction
Pleural mesothelioma is a cancer that typically starts in cells lining of the chest (1). Malignant mesothelioma is relatively rare (2-6). However, it is a highly malignant tumor, which arises from the mesothelial cells of the pleura, peritoneum, pericardium, and tunica vaginalis (1). More than 80% of malignant mesothelioma patients have histories of asbestos-exposure (1,7-9). Incubation period between the onset of malignant mesothelioma and asbestos exposure is about 30–40 years (7,8). The incidence of malignant mesothelioma would be expected to increase gradually in the world due to the asbestos exposures in each county after the World War II, mishandling of environmental carcinogens, ion radiation, viruses and genetic factors (1,8,10). Early detection of malignant pleural mesothelioma (MPM) is usually difficult due to its asymptomatic and non-specific presentation characters (5,7).
Typical radiologic studies, such as X-ray and CT scan, show unilateral pleural effusion and/or a diffuse pleural thickening or masses for typical MPM cases (11-14). However, it may manifests in a benign localized form such as benign localized fibrous tumor (BLFT) or solitary fibrous tumors of pleural (SFTP) (2,3,15-20). Furthermore, malignant mesothelioma has a wide histopathologic spectrum of various cell types (21). This makes it difficult to differentiate from other pleural tumors using only hematoxylin-eosin staining and immunohistochemistry to make diagnosis of malignant mesothelioma (7,21-23).
In this paper, we report a case of localized malignant pleural sarcomatoid mesothelioma (SM), which mimics as a BLFT of the pleura on the initial radiologic and pathologic examination.
Case presentation
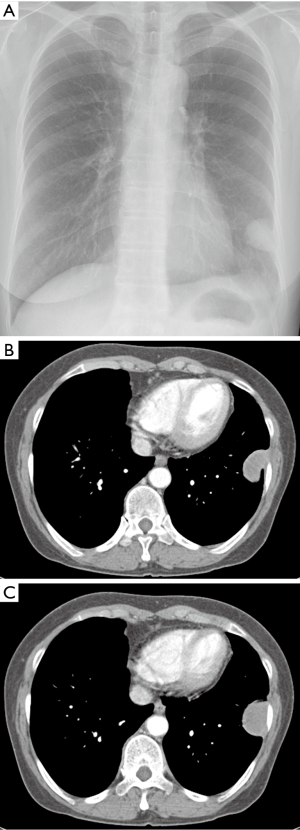
A 65-year-old asymptomatic female presented with a mass on chest X-ray. Patient had no past history of exposing to carcinogenic chemical known as asbestos. Her past medical history was unremarkable. There was no cancer history in her family. Her initial chest X-ray was taken and showed a sharp circumscribed mass in the left lower lung area abutting the lateral chest wall (Figure 1A). The contrast-enhanced CT scan performed and revealed a sharp homogenous oval enhancing mass with the diameter of 3.5 cm abutting the pleura in the upper left of the chest wall (Figure 1B,C). The tumor showed smooth margins and pedunculation. Pleural effusion and or other pleural abnormalities were not observed in the thorax. Percutaneous needle biopsy of the mass was performed with an 18-guage automated cutting needle. Pathologic examination revealed fibrocollagenous tissue with no evidence of malignancy. Then, the complete surgical excision of the mass was performed for histopathology analysis. The surgical specimen showed a benign fibroblast proliferation with collagenous fibrotic background. Plus, tumor cells were negative for calretinin, CD34, actin, and CK20 (Calretinin is a positive marker for malignant mesothelioma. CD 34 is a positive marker for solitary fibrous tumor. Actin is a positive marker for desmoid cyst). However, tumor cells had focal weak staining positive for TTF-1 and CK7 (TTF-1 is a negative marker for malignant mesothelioma). From the immunohistochemistry, differential diagnosis was a demoid and solitary fibrous tumor of the pleura (SFTP); specifically, it was rather a BLFT of the pleura.
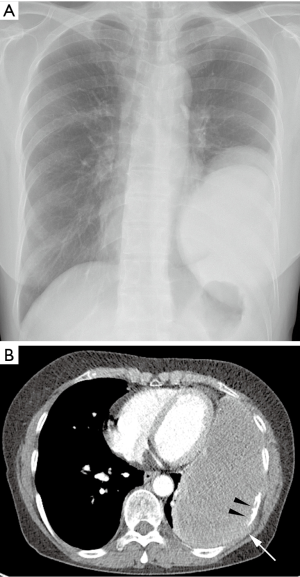
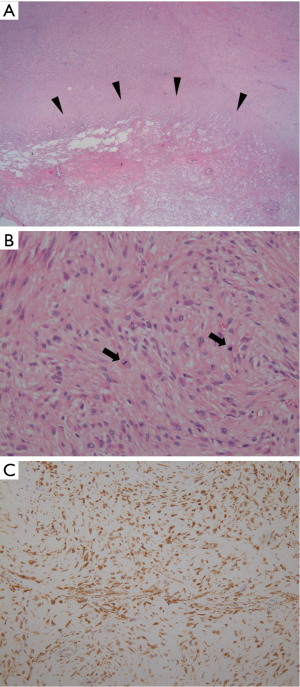
Seven months after the surgery, patient visited our hospital with new complaints of progressive dyspnea and chest pain. A follow-up chest X-ray was taken and showed a large recurrent mass at the site of previous mass excision (Figure 2A). Contrast-enhanced CT scan was obtained and revealed a large homogeneously enhancing mass in the left lower hemi-thorax with invasion to its adjacent ribs and chest wall (Figure 2B). Large pleural effusion was observed. There was no evidence of metastasis to the thorax and upper abdomen. The second percutaneous needle biopsy was performed from the mass. Histopathologic examination revealed a diffuse spindle cell proliferation with mild pleomorphism and frequent appearance of mitosis in the collagenous background (Figure 3A,B). In addition, tumor cells showed positive for D2-40, which is a novel mesothelial positive marker for malignant mesothelioma (Figure 3C). Therefore, this confirmed the diagnosis of SM, a subtype of MPM. A second surgery was done to remove the mass. Pleurectomy and left lower lobectomy were performed with partial removal of affect adjacent ribs and a part of affect diaphragm, which were invaded by the tumor. Patient was also treated with chemotherapy and radiation therapy. Treatment was successful without mortality. Patient was discharged home and returned to normal life.
Discussion
Malignant mesothelioma is a common pleural neoplasm due to asbestos exposure for 80% of cases (1,7,8). Its prognosis is poor (5,7). Localized malignant pleural mesothelioma (LMPM) is a rare tumor (2-6). The incidence is 1.25/100,000 in Great Britain and 1.1/100,000 in Germany (4). In the United States, about 3,000 new mesothelioma cases have been diagnosed each year (1,24). Only a small number of case reports have been published in English literature (2) since Crotty et al. first described a series of six localized malignant mesotheliomas in 1994 (3). Most, but not all localized malignant mesotheliomas, were of pleural origin (21). LMPMs appeared as solitary circumscribed nodule or mass attached, either in a sessile or pedunculated manner to the surface of the pleura (25). This type of tumor should be distinguished from diffuse MPMs, because a good prognosis may be obtained by surgical resection (2,15,16).
Malignant mesothelioma has a wide histopathologic spectrum of various cell types. Although it has three typical cell types, which are epithelioid, sarcomatoid, and biphasic, epithelioid are common and tend to have better prognosis than the other types (1). About 10% of mesotheliomas are sarcomatoid (1), which has worse outcome (7). In the past few years, an accurate differentiation between subtypes of MPM has been challenging due to differences in chemosensitivity and clinical presentation (5,7). There are atypical variations, from lympho histiocytoid, small cell, deciduoid, clear cell, to other benign pleomorphic types such as localized fibrous tumor of the pleura (LFTP) and solitary fibrous of tumor of the pleura (SFTP). LFTP is also a rare benign tumor and is asymptomatic in half the patients (17). The diagnosis is difficult to establish before operation (17). Also, it is difficult to diagnose solely on immunohistochemistry and microscopic examination, which are the gold standard tools to diagnosis malignant mesothelioma (4,7). Today, there are positive and negative markers in immunohistochemistry to help make differential diagnosis of mesothelioma (22,23,26). Main positive markers are as calretinin, CK5/6, Wilms tumor 1 protein (WT1), D2-40, podoplanin, and mesothelin (21,26). Main negative markers are TTF-1, CEA, MOC-31, B72.3, and Ber-EP4 (21).
In the presented case, the initial radiologic finding was a single 3.5cm diameter pleural mass. Microscopic study found spindle cell proliferation favored fibrous tumor, such as solitary fibrous tumor, localized fibrous tumor, or desmoid tumor, which commonly occurs in the pleura. Therefore, pathologist performed immunohistochemistry for only calretinin, which is the most commonly used marker for malignant mesothelioma. Unfortunately, tumor cells were negative for calretinin. Plus, the patient was asymptomatic. Overall, this supported the initial differential diagnosis of LFTP (17). However, seven months later patient returned with worse progress, which displayed mesothelioma symptoms such as progressive dyspnea and chest pain. Now, radiographic studies showed a solitary large well-defined homogeneous re-current mass in the left lower hemithorax at the old resection sites seven months ago. There were sign of bony destruction of adjacent rib and chest wall due to tumor invasion. Pleural malignancy was highly suspected. Immunohistochemistry of tumor markers for malignant mesothelioma were scanned. Tumor cells were positive for D2-40, which confirmed the diagnosis for malignant pleural SM (26).
Malignant pleural SM usually presents with unilateral pleural effusion or diffuse pleural thickening or masses (27). However, atypical presentation, such as LMPM, would be expected to be frequent, along with an increase of the overall incidence of malignant mesothelioma (10). This would be expected to increase until the predicted peak year; 2015–2020 in Europe, 2025 in Japan, and 2015 in Australia (10,28). The important fact is most LMPM can be resolved by surgical excision (29-33). Therefore, physicians should attempt pathological examination as early as possible to prevent tumor invasion, even when the mass is localized and tiny. A careful pathologic examination should be performed with immunohistochemistry using more than two positive markers for malignant mesothelioma. The combination of CAM5.2, WT1, and AE1/AE3 are recommended for routine pathological diagnosis (22). A Japanese study by Kushitani et al. in 2008 showed that CAM5.2 had the highest sensitivity and specificity for differentiating SM (22).
The presented case showed a huge recurrent mass, several months after a surgical resection; whereas, some previously reported LMPM were cured by a surgical resection (2,15,16). Subtype of the presented case was sarcomatoid cell type (7,8). It is the least common of the three mesothelioma cell types. It can be challenging to diagnose (8) because it tends to resemble fibrosarcomas, or malignant soft tissue tumors in the fibrous connective tissue. SM cells are more resistant than the other cell types to most treatments, including surgery, chemotherapy (34-36) and radiation therapy, which has the poorest prognosis (37,38).
In conclusion, localized malignant pleural SM may radiologically and pathologically mimic benign fibrous tumors arising from the pleura, such as solitary fibrous tumor or localized fibrous tumors. As the incidence of malignant mesothelioma gradually increase in the world, a careful pathological examination should be performed to differentiate malignant mesothelioma from benign tumor of the mesothelium. This should be done even when a mass is localized and small on the radiologic studies even when patient is asymptomatic.
Acknowledgements
Funding: This work was supported by a National Research Foundation of Korea Grant (NRF-2011-0014570).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- American Cancer Society. Malignant Mesothelioma. 2015 Copyright American Cancer Society. Available online: www.cancer.org/malignant-mesothelioma-pdf
- Nakano T, Hamanaka R, Oiwa K, et al. Localized malignant pleural mesothelioma. Gen Thorac Cardiovasc Surg 2012;60:468-74. [Crossref] [PubMed]
- Crotty TB, Myers JL, Katzenstein AL, et al. Localized malignant mesothelioma. A clinicopathologic and flow cytometric study. Am J Surg Pathol 1994;18:357-63. [Crossref] [PubMed]
- Stahel RA, Weder W, Lievens Y, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v126-8. [Crossref] [PubMed]
- Makimoto G, Fujiwara K, Fujimoto N, et al. Phrenic nerve paralysis as the initial presentation in pleural sarcomatoid mesothelioma. Case Rep Oncol 2014;7:389-92. [Crossref] [PubMed]
- Tanzi S, Tiseo M, Internullo E, et al. Localized malignant pleural mesothelioma: report of two cases. J Thorac Oncol 2009;4:1038-40. [Crossref] [PubMed]
- Galetta D, Catino A, Misino A, et al. Sarcomatoid mesothelioma: future advances in diagnosis, biomolecular assessment, and therapeutic options in a poor-outcome disease. Tumori 2016;102:127-30. [Crossref] [PubMed]
- Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Semin Oncol 2002;29:2-17. [Crossref] [PubMed]
- Pan XL, Day HW, Wang W, et al. Residential proximity to naturally occurring asbestos and mesothelioma risk in California. Am J Respir Crit Care Med 2005;172:1019-25. [Crossref] [PubMed]
- Robinson BW, Lake RA. Advances in Malignant Mesothelioma. N Engl J Med 2005;353:1591-603. [Crossref] [PubMed]
- Zhou H, Tamura T, Kusaka Y, Suganuma N, et al. Development of a guideline on reading CT images of malignant pleural mesothelioma and selection of the reference CT films. Eur J Radiol 2012;81:4203-10. [Crossref] [PubMed]
- Sahin AA, Cöplü L, Selçuk ZT, et al. Malignant pleural mesothelioma caused by environmental exposure to asbestos or erionite in rural Turkey: CT findings in 84 patients. AJR Am J Roentgenol 1993;161:533-7. [Crossref] [PubMed]
- Wang ZJ, Reddy GP, Gotway MB, et al. Malignant pleural mesothelioma: evaluation with CT, MR imaging, and PET. Radiographics 2004;24:105-19. [Crossref] [PubMed]
- Kawashima A, Libshitz HI. Malignant pleural mesothelioma: CT manifestations in 50 cases. AJR Am J Roentgenol 1990;155:965-69. [Crossref] [PubMed]
- Takahashi H, Harada M, Maehara S, et al. Localized malignant mesothelioma of the pleura. Ann Thorac Cardiovasc Surg 2007;13:262-6. [PubMed]
- Turna A, Pekçolaklar A, Fener N, et al. Localized malignant pleural mesothelioma treated by a curative intent lobectomy: a case report. Ann Thorac Cardiovasc Surg 2007;13:349-51. [PubMed]
- Suter M, Gebhard S, Boumghar M, et al. Localized fibrous tumour of the pleura: 15 new cases and review of literature. Eur J Cardiothorac Surg 1998;14:453-9. [Crossref] [PubMed]
- Enon S, Kilic D, Yuksel C, et al. Benign localized fibrous tumor of the pleura: report of 25 new cases. Thorac Cardiovasc Surg 2012;60:468-73. [Crossref] [PubMed]
- Yeom YK, Kim MY, Lee HJ, et al. Solitary Fibrous Tumors of the Pleura of the Thorax: CT and FDG PET Characteristics in a Tertiary Referral Center. Medicine (Baltimore) 2015;94:e1548. [Crossref] [PubMed]
- Harrison-Phipps KM, Nichols FC, Schleck CD, et al. Solitary fibrous tumors of the pleura: results of surgical treatment and long-term prognosis. J Thorac Cardiovasc Surg 2009;138:19-25. [Crossref] [PubMed]
- Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647-67. [Crossref] [PubMed]
- Kushitani K, Takeshima Y, Amatya VJ, et al. Differential diagnosis of sarcomatoid mesothlioma from sarcoma and sarcomatoid carcinoma using immunohistochemistry. Pathol Int 2008;58:75-83. [Crossref] [PubMed]
- Takeshima Y, Amatya VJ, Kushitani K, et al. Value of immunohistochemistry in the differential diagnosis of pleural sarcomatoid mesothelioma from lung sarcomatoid carcinoma. Histopathology 2009;54:667-76. [Crossref] [PubMed]
- Price B, Ware A. Mesothelioma trends in the United States: An update based on Surveillance Epidemiology and End Results program date for 1973-2003. Am J Epidemiol 2004;159:107-12. [Crossref] [PubMed]
- Haithcock BE, Zagar TM, Zhang L, et al. Chapter 73: Diseases of the pleura and mediastinum. In: Niederhuber JE, Armitage JO, Dorshow JH, et al. editors. Abeloff’ s Clinical Oncology. 5th ed. Philadelphia, Pa. Elsevier, 2014.
- Chu AY, Litzky LA, Pasha TL, et al. Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol 2005;18:105-10. [Crossref] [PubMed]
- Klebe S, Brownlee NA, Mahar A, et al. Sarcomatoid mesothelioma: a clinical-pathologic correlation of 326 cases. Mod Pathol 2010;23:470-9. [Crossref] [PubMed]
- Pelucchi C, Malvezzi M, La Vecchia C, et al. The mesothelioma epidemic in Western Europe: an update. Br J Cancer 2004;90:1022-4. [Crossref] [PubMed]
- Bölükbas S, Schirren J. Malignant pleural mesothelioma: comparison of radical pleurectomy und extrapleural pneumonectomy. Chirurg 2013;84:487-91. [PubMed]
- Schipper PH, Nichols FC, Thomse KM, et al. Malignant pleural mesothelioma: surgical management in 285 patients. Ann Thorac Surg 2008;85:257-64; discussion 264. [Crossref] [PubMed]
- Allen KB, Faber LP, Warren WH. Malignant pleural mesothelioma. Extrapleural pneumonectomy and pleurectomy. Chest Surg Clin N Am 1994;4:113-26. [PubMed]
- Wolf AS, Daniel J, Sugarbaker DJ. Surgical techniques for multimodality treatment of malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:132-48. [Crossref] [PubMed]
- Sugarbaker DJ, Wolf AS. Surgery for malignant pleural mesothelioma. Expert Rev Respir Med 2010;4:363-72. [Crossref] [PubMed]
- Alexander HR Jr, Bartlett DL, Pingpank JF, et al. Treatment factors associated with long- term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013;153:779-86. [Crossref] [PubMed]
- Arrieta Ó, Medina LA, Estrada-Lobato E, et al. First-line chemotherapy with liposomal doxorubicin plus cisplatin for patients with advanced malignant pleural mesothelioma: phase II trial. Br J Cancer 2012;106:1027-32. [Crossref] [PubMed]
- Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy- resistant advanced malignant mesothelioma: An open-label, single-arm, phase 2 trial. Lancet Oncol 2013;14:1104-11. [Crossref] [PubMed]
- Rimner A, Rosenzweig KE. Novel radiation therapy approaches in malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:457-61. [PubMed]
- Gupta V, Krug LM, Laser B, et al. Patterns of local and nodal failure in malignant pleural mesothelioma after extrapleural pneumonectomy and photon-electron radiotherapy. J Thorac Oncol 2009;4:746-50. [Crossref] [PubMed]


