Tumor-to-tumor metastasis: an unusual case of breast cancer metastatic to a solitary fibrous tumor
Introduction
Solitary fibrous tumors (SFTs) are rare mesenchymal neoplasms that most commonly involve the visceral or parietal pleura. These tumors account for less than 5% of all pleural tumors (1). Extrapleural locations have been described arising from a wide variety of anatomic locations, including the nasopharynx, bladder, prostate, soft tissue of the neck, buttock, extremities, and abdominal wall (2). We present an unusual case of a tumor-to-tumor metastasis involving breast cancer as the donor to a SFT recipient diagnosed six years after definitive treatment of the primary breast cancer.
Case presentation
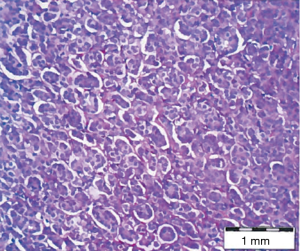
The patient is a 64-year-old woman who was diagnosed with her third primary cancer over an 8-year period. In 2004, she was diagnosed with diffuse large B-cell lymphoma of the thyroid gland, for which she received four cycles of rituximab (Rituxan®, Genentech, San Francisco, CA, USA) and CHOP [cyclophosphamide (Cytoxan®, Bristol-Myers-Squibb, Princeton, NJ, USA), hydroxyl-daunorubicin (Adriamycin®, Pfizer, New York, NY, USA), vincristine (Oncovin®, Eli Lilly, Indianapolis, IN, USA), prednisone] chemotherapy, followed by radiation including upper mantle, as well as the thyroid grand. In 2006, she developed a right axillary mass, which on further workup was consistent with a primary right breast cancer. She received neoadjuvant chemotherapy consisting of three cycles of doxorubicin (Adriamycin®) and cyclophosphamide, followed by right modified radical mastectomy and a prophylactic left mastectomy. Final pathology demonstrated residual invasive ductal carcinoma, Nottingham Grade I, estrogen-receptor (ER)-positive, progesterone-receptor (PR)-positive, HER2/neu-negative, with 9 out of 11 lymph nodes positive for metastatic disease, T1cN2aM0 (stage IIIA) (Figure 1). Postoperatively, she received 12 cycles of paclitaxel (Taxol®, Bristol-Myers-Squibb) as adjuvant chemotherapy and 5 years of hormonal therapy with letrozole (Femara®, Novartis Oncology, East Hanover, NJ, USA).
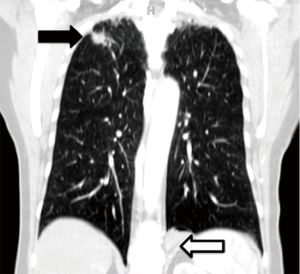
In 2012, she presented to thoracic surgery clinic with shortness of breath and a non-productive cough. Computerized tomography (CT) scan of the thorax revealed bilateral pulmonary lesions (Figure 2). In the right lung apex was a 2.2 cm × 1.1 cm × 1.6 cm spiculated mass, with adjacent scarring and pleural thickening. Morphologic characteristics were concerning for a primary bronchogenic neoplasm. In the postero-medial left lung base was a 3-cm mass adjacent to the left hemidiaphragm and left paravertebral gutter.
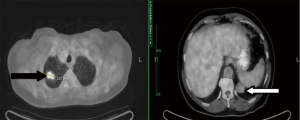
A positron-emission tomography (PET)-CT scan showed hypermetabolic activity in the spiculated right upper lung lesion, with a maximum standardized uptake value (maxSUV) of 7.6 (Figure 3). There was slightly increased activity in a pre-carinal lymph node. The left lower lobe lesion was not fluoro-deoxy-glucose (FDG)-avid. Transthoracic core-needle biopsy of the left lung mass was non-diagnostic.
The patient underwent video-assisted thoracoscopic left lower lobe wedge resection, immediately followed by robotic-assisted video-thoracoscopic right upper lobectomy with mediastinal lymph node dissection. Total operative (skin-to-skin) time was 267 min; estimated blood loss was 500 mL. She had no postoperative complications. The left chest tube was removed on postoperative day (POD) #1; the right chest tube was removed on POD#4. She was discharged home on POD#4.
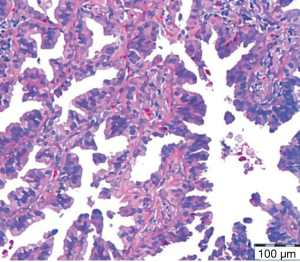
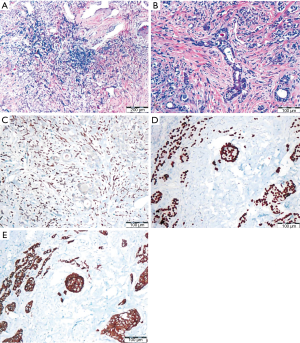
Final pathology revealed a 1.4 cm × 1.3 cm × 0.8 cm right upper lobe moderately-differentiated adenocarcinoma with peripheral bronchioloalveolar pattern, T1aN0M0, stage 1A (negative resection margins) (Figure 4). Immunohistochemical stains displayed tumor cells positive for thyroid transcription factor-1 (TTF-1), supporting lung origin for the adenocarcinoma. Pathology review described the left lung lesion as a 4 cm × 3.5 cm × 1.8 cm pedunculated pleural cystic structure with mural nodules of SFT admixed with foci of metastatic invasive ductal breast carcinoma, ER/PR-positive, extending to within 0.5 mm of the SFT surface and present within vessels of the pseudocapsule. Wedge resection margins were free of tumor. Breast tumor morphology reveals tubules, solid nests/tubules and a single cell lobular pattern. An immunohistochemical stain for STAT6 reveals diffuse nuclear positivity in the spindle cells, and absence of staining in adjacent breast carcinoma confirming the diagnosis of SFT (Figure 5A-C). Breast tumor epithelial cells reveal nuclear positivity for GATA-3 and membranous staining for e-cadherin in all patterns (Figure 5D,E). The resected primary breast mass is not available to examine for these patterns, but stains confirm metastatic breast carcinoma.
The patient has been on long-term systemic hormonal therapy with exemestane (Aromasin®, Pfizer) and fulvestrant (Faslodex®, AstraZeneca, Wilmington, DE, USA) monthly injections and is currently with no evidence of disease.
Discussion
This case report presents a breast cancer 6-year survivor who was found to have a SFT harboring metastatic breast cancer. We compare our case with the five other cases of tumor-to-tumor metastasis to a SFT described in the literature (3-7) (Table 1).
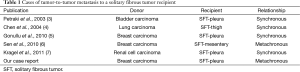
Full table
Tumor-to-tumor metastasis is an unusual phenomenon. According to Campbell et al., tumor-to-tumor metastasis must meet the following criteria: (I) more than one primary tumor must exist; (II) the recipient tumor is a true benign or malignant neoplasm; (III) the metastatic neoplasm is a true metastasis with established growth in the host tumor, not the result of contiguous growth or embolization of tumor cells; and (IV) tumors which have metastasized to the lymphatic system, where lymphoreticular malignant tumors already exist, are excluded (8).
Renal cell carcinoma is the most common recipient for tumor-to-tumor metastasis, followed by breast cancer (9). A SFT is an extraordinarily rare recipient for tumor-to-tumor metastasis that has now been described in six cases, including the present case. Macroscopically, a SFT appears as a firm, smooth, lobulated mass surrounded by a vascular rich capsule. They are characterized microscopically by an erratic organization of spindle cells intertwining with connective tissue, referred as a “patternless pattern”. By definition, SFT are vimentin-positive, keratin-negative, with CD34/bcl-2 expression (10). STAT6 expression is diagnostic. SFTs can potentially arise from either intra- or extrathoracic sources. However, SFT most frequently arises from intrathoracic sources. This distribution was also seen in our literature review of tumor-to-tumor metastatic to SFT: four of the six cases were intrathoracic, one intraabdominal, and one from soft tissue of the thigh.
SFTs exhibit rich vascularity, similar to renal cell carcinoma, making them especially suitable for harboring metastasis. Breast cancer was the most common metastatic donor to a SFT in our review of literature. Four of the six cases involved synchronous donor cancer lesions. The other two cases, including the present case, had metachronous primary and metastatic cancers, with 3 and 6 years between diagnoses, respectively. Interestingly, even though the primary breast cancer was diagnosed six years before the SFT in this case, this patient also had a contralateral primary pulmonary adenocarcinoma synchronous with the breast metastasis in the SFT. The phenomenon may become more frequent because of the improving prognosis and survival of patients with primary malignancies, including breast cancer patients such as that in the present case (11).
PET scan has a reported average sensitivity and specificity of 96% and 77%, respectively, for detecting breast cancer metastatic lesions. Limitations of FDG-PET in breast cancer patients include the relatively low detection rate of bone metastases and the relatively high rate of false-positive results (12). However, our case had a PET-negative breast metastatic lesion, and, by patient report, the primary breast cancer was also PET-negative. Aggressive workup and subsequent resection of a PET-negative, core-needle biopsy-negative lung mass helped to provide the adequate treatment for this patient.
Therapeutic advances in breast cancer have increased the survival rate of breast cancer patients. Therefore, breast cancer survivors face an increased risk for developing a second primary cancer. Lung cancer represents 5% of these second primary cancers (13). Diagnosis of breast cancer before 50 years of age and a history of radiation therapy exposure are two risk factors for a breast cancer patient to develop a second primary cancer in the lung. Our patient had history of radiation therapy due to her lymphoma.
Our case report discusses the rare entity of tumor-to-tumor metastasis, especially into a SFT as recipient. Our case report also emphasizes that PET imaging is not necessarily helpful in the routine surveillance of breast cancer patients and that continued work-up of a lung abnormality that is suspicious for malignancy based on clinical history and imaging is needed despite a negative PET scan and non-diagnostic core needle biopsy.
Acknowledgements
The authors would like to acknowledge Carla C. Moodie, PA-C, Joseph R. Garrett, ARNP-C, MPH, and Nazanin I. Khakpour, MD, for their help in the care of this patient.
Footnote
Conflicts of Interest: EM Toloza has a financial relationship with Intuitive Surgical Corporation in form of honoraria as robotic thoracic surgery proctor and observation site. Previously presented as a Poster Presentation at the Chest World Congress 2014 in Madrid, Spain, on March 21–24, 2014.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Lu C, Ji Y, Shan F, et al. Solitary fibrous tumor of the pleura: an analysis of 13 cases. World J Surg 2008;32:1663-8. [Crossref] [PubMed]
- Mentzel T, Bainbridge TC, Katenkamp D. Solitary fibrous tumour: clinicopathological, immunohistochemical, and ultrastructural analysis of 12 cases arising in soft tissues, nasal cavity and nasopharynx, urinary bladder and prostate. Virchows Arch 1997;430:445-53. [Crossref] [PubMed]
- Petraki C, Vaslamatzis M, Argyrakos T, et al. Tumor to tumor metastasis: report of two cases and review of the literature. Int J Surg Pathol 2003;11:127-35. [Crossref] [PubMed]
- Chen HW, Dry SM, Seeger LL. Primary lung carcinoma metastatic to a solitary fibrous tumor. Skeletal Radiol 2004;33:226-9. [Crossref] [PubMed]
- Gonullu G, Sullu Y, Basoglu A, et al. Metastatic breast carcinoma to solitary fibrous tumor in the lung. Indian J Cancer 2010;47:76-8. [Crossref] [PubMed]
- Sen S, Menon S, McCulloch T. Mesenteric solitary fibrous tumor containing metastasis from breast carcinoma: an unusual example of tumor-to-tumor metastasis. Diagn Histopathol 2010;16:397-9. [Crossref]
- Kragel C, Wei S. Renal cell carcinoma metastasizing to solitary fibrous tumor of the pleura: a case report. J Med Case Rep 2011;5:248. [Crossref] [PubMed]
- Campbell LV Jr, Gilbert E, Chamberlain CR Jr, et al. Metastases of cancer to cancer. Cancer 1968;22:635-43. [Crossref] [PubMed]
- Sella A, Ro JY. Renal cell cancer: best recipient of tumor-to-tumor metastasis. Urology 1987;30:35-8. [Crossref] [PubMed]
- de Perrot M, Fischer S, Bründler MA, et al. Solitary fibrous tumors of the pleura. Ann Thorac Surg 2002;74:285-93. [Crossref] [PubMed]
- Shin T, Kan T, Sato F, et al. Tumor-to-Tumor Metastasis to Chromophobe Renal Cell Carcinoma: A First Report. Case Rep Urol 2011;2011:520839.
- Lind P, Igerc I, Beyer T, et al. Advantages and limitations of FDG PET in the follow-up of breast cancer. Eur J Nucl Med Mol Imaging 2004;31 Suppl 1:S125-34. [Crossref] [PubMed]
- Schonfeld SJ, Curtis RE, Anderson WF, et al. The risk of a second primary lung cancer after a first invasive breast cancer according to estrogen receptor status. Cancer Causes Control 2012;23:1721-8. [Crossref] [PubMed]