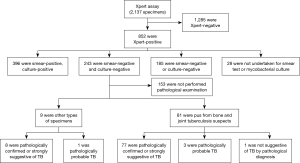
The reliability analysis of Xpert-positive result for smear-negative and culture-negative specimen collected from bone and joint tuberculosis suspects
Introduction
Tuberculosis (TB) has been a leading cause of death worldwide for decades, and China is one of the 22 countries with the highest burden of the disease (1). TB control depends on accurate identification of cases and prompt treatment, thereby interrupting transmission chains and reducing morbidity. In China, finding the TB cases largely relies on the acid-fast bacilli (AFB) smear microscopy because it is rapid and easily applied, while the diagnosis is also carried out by the conventional solid or broth culturing. However, low sensitivity of smear testing and the time consuming characterization of culture make them suboptimal for TB diagnosis (2-5).
Xpert MTB/RIF (Xpert; Cepheid, Sunnyvale, CA, USA) assay, a new and automated molecular test, can detect the presence of mycobacterium tuberculosis complex (MTC)-specific rpoB gene sequence as well as the rifampicin resistance gene mutations in 2 hours (6). The Xpert assay initially endorsed by the World Health Organization (WHO) in 2010 for pulmonary TB diagnosis due to excellent performance for detecting both pulmonary and extrapulmonary tuberculosis (EPTB) specimen (7), but the scope of application expanded to all types of TB by 2013 (8). A recent study on the sputum specimens demonstrated that Xpert obtained 96.7% (88/91) sensitivity for smear-positive and culture-positive specimens, 44.3% (43/97) for smear-negative but culture-positive specimens, and 16.8% (31/185) for smear-negative and culture-negative specimens (9).
Various independent evaluations of Xpert MTB/RIF have largely focused on comparisons between Xpert, smear test and mycobacterial culture while not taking into account the reliability of Xpert when bacteriological examination was inconsistent. However, in clinical practice, smear-and culture-negative but Xpert-positive specimens are frequently confronted. Such Xpert assay results require further characterizations for assessing whether the results are not a false positive or do not have nucleic acid contaminations which affect the final result. To clarify the ambiguities associated with these clinical results, that laboratories often present, we performed a retrospective study for analyzing the reliability of Xpert-positive results for smear-negative and culture-negative specimens from TB suspects.
Methods
Study design
Xpert assay was carried out for a total of 2,137 specimens from April 2014 to February 2015 at the Beijing Chest Hospital (Beijing, China). The eligibility criterion in this study was as follows: (I) Xpert assay, AFB smear microscopy, Mycobacterium culturing and pathological examination were performed using same specimen or on specimens collected from same clinical operations; (II) the specimens were Xpert-positive, smear-negative and culture-negative. Since this study was a laboratory based retrospective study, the ethics approval was waived by the Beijing Chest Hospital Ethic Committee.
Microscopy examination, culture and Xpert assays
Tissue specimens were homogenized in a 2 mL phosphate with Fastprep-24 automated homogenizer (MP Company, USA) whereas other specimens were directly processed. Staining was performed by using auramine and the smears were then examined by light-emitting diode (LED) microscopy. The smears were read and interpreted in accordance with WHO guidelines (10). The solid culture with Lowenstein-Jensen (LJ) medium was also performed following guidelines from WHO (11).
The unprocessed specimens and the homogenized biopsy tissues were tested by Xpert MTB/RIF according to manufacturer’s instructions. Briefly, the sample reagent was added in a 2:1 ratio per 1 mL of the specimens, vortexed for at least 10 s, and then incubated for 10 min at room temperature. The mixture was vortexed again for another 10 s and incubated at room temperature for 5 min. About 2 mL of the mixture was transferred into the Xpert cartridge and loaded into the Xpert instrument and the automatic detection procedure was performed.
Pathological examination
The tissue specimens were fixed in neutral formalin, dehydrated and subsequently paraffin-embedded. Corresponding to the bronchoalveolar lavage fluid (BALF) samples, which were examined by Xpert, biopsy tissue collected during same bronchoscope operations were used to carry out pathological examinations. Sediments from 50 mL pleural effusion samples, after centrifugation at 3,000 rpm for 10 min, were also fixed in formalin and embedded in paraffin. The 4 µm sections were stained with haematoxylin and eosin solution and observed by light microscopy forpatho-morphological changes. AFB test was performed only when the pathologists needed further information. Briefly, 4.0 µm sections were dewaxed by dimethylbenzene, then sequentially washed with 95%, 90%, 85%, 70% ethanol, and finally by de-ion water. After drying, the slides were stained by standard Zeihl-Neelsen method, and AFB was detected under oil immersion lens (×1,000).
The pathological diagnostic criteria included: (I) Confirmed TB: AFB was observed in the lesion, chronic granulomatous inflammation, with or without caseous necrosis, were also observed; (II) probable TB: typical chronic granulomatous inflammation with caseous necrosis were observed, AFB was not observed or the test was not performed; (III) possible TB: chronic inflammation or caseous necrosis were observed, AFB was not observed; (IV) no TB: neither granulomatous inflammation nor caseous necrosis were observed.
Results
From a total of 2,137 specimens prescribed Xpert MTB/RIF assays, 852 (40%) were Xpert-positive. Ultimately, 90 specimens from 90 TB suspects were enrolled in this study according to the eligibility criterion (Figure 1). Of these, 49% (44/90) were female and 51% (46/90) were male, the median age was 35.94 yr with an age range of 3 to 81 yr, 12% (11/90) were children and 88% (79/90) were adults.
The recruited specimens consisted of 81 pus specimens collected from Bone and Joint Tuberculosis (BJTB) patients during debridement operations, 5 BALF, 3 pleural fluids, and 1 pulmonary biopsy tissue. 56 specimens taken for performing AFB tests, with paraffined specimens, 77% (43/56) of them were AFB positive.
According to the pathological examination results, 77 of the 81 pus specimens, 8 of 9 other types of specimens were confirmed as either TB or strongly suggestive of TB; three pus specimens and one biopsy tissue were also suggested TB but with less stronger evidence; only one pus specimen was not TB suggestive. Rifampicin resistance was detected in 14% (13/90) of the recruited cases.
Discussion
In countries with high TB prevalence, such as China, conventional diagnostic tools are commonly used for case finding. Usually if both the smear and culture test produce negative results for the TB patients, diagnosis is postponed which increases the chances of disease transmission. Recent advances in molecular diagnostic techniques have improved both the TB diagnosis and also the drug susceptibility tests, whereas the possibility of contamination still remains a concern. The Xpert MTB/RIF assay is an automated molecular test that simultaneously detects M. tuberculosis and rifampicin resistance. Sufficient validations have been done for the smear test and/or the culture confirmed TB, whereas for no bacteriologically-confirmed TB, further evaluations are still needed. In this retrospective study, the absolute majority of the recruited smear- and culture-negative but Xpert-positive specimens collected from BJTB patient suspects were finally diagnosed as TB by pathological examinations, which indicated that Xpert positive was highly reliable even when the smear test and culture produced negative results for the same clinical specimen. Among all recruited specimens, 14% (13/90) were diagnosed as rifampicin-resistant, which is a reliable indicator of MDR-TB. Therefore, without Xpert assay, the diagnosis of MDR-TB for these specimens would not have been accomplished.
We also tried to get additional confirmation for the true performance of Xpert assay for specimens without relevant pathological examination. Therefore, the outcomes of bacteriological examinations from different specimens or from the specimens collected during different clinical operations of the excluded 153 specimens with Xpert-positive but smear-negative and culture-negative outcomes from this study were also analyzed. We found that 18 specimens were smear-positive, 5 specimens were culture-positive and 1 specimen was smear-positive and culture-positive.
There are some limitations of this study. Firstly, the study was carried out in a TB designated hospital, the conclusion under same scenario but low TB prevalence setting may be different. Secondly, the types and the number of some specimens involved in the study were limited. About 90% (81/90) of the recruited specimens were pus specimens collected from bone and joint TB suspects. Other study reported that among all kinds of EPTB specimens, the highest yield of Xpert positivity was presented by pus samples of BJTB patients (12). Our previous work also demonstrated that pus specimens of BTJB patients have high positive rate (13). Thirdly, we did not consider the possibility of non-tuberculous mycobacteria (NTM) during the pathological examinations. Located in southern China, Beijing is a low prevalence territory for NTM infection, and our previous work suggested that NTM was of little importance for BJTB patients (14).
In summary, our study showed that Xpert-positive result was trustable for BJTB diagnosis even when the bacteriological examination did not produce consistent results. Our assay may reduce confusion of clinicians in such scenario.
Acknowledgements
Funding: The work was supported by the research funding from Infectious Diseases Special Project, Ministry of Health of China (2012ZX10003002-009) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201304).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization. Global Tuberculosis Report 2013. Geneva, Switzerland. WHO/HTM/. TB/2013.11.
- Siddiqi K, Lambert ML, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis 2003;3:288-96. [Crossref] [PubMed]
- Young DB, Perkins MD, Duncan K, et al. Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest 2008;118:1255-65. [Crossref] [PubMed]
- Sun JR, Lee SY, Perng CL, et al. Detecting Mycobacterium tuberculosis in Bactec MGIT 960 cultures by inhouse IS6110-based PCR assay in routine clinical practice. J Formos Med Assoc 2009;108:119-25. [Crossref] [PubMed]
- Zhao P, Fang F, Yu Q, et al. Evaluation of BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to first-line drugs in China. PLoS One 2014;9:e99659. [Crossref] [PubMed]
- Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005-15. [Crossref] [PubMed]
- World Health Organization. Roadmap for rolling out Xpert MTB/RIF for rapid diagnosis of TB and MDR-TB; 2010. Geneva, Switzerland: WHO, 2013. Available online: www.who.int/tb/laboratory/roadmap_xpert_mtb-rif.pdf
- World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Geneva: World Health Organization. 2013.
- Huang F, Dang L, Sun H, et al. A study of the value of three molecular diagnostic techniques in the diagnosis of tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi 2015;38:680-5. [PubMed]
- International Union Against Tuberculosis and Lung Disease. TECHNICAL GUIDE: Sputum Examination for Tuberculosis by Direct Microscopy in Low Income Countries. 5th ed. Paris: International Union against Tuberculosis and Lung Disease, 2000.
- Chihota VN, Grant AD, Fielding K, et al. Liquid vs. solid culture for tuberculosis: performance and cost in a resource-constrained setting. Int J Tuberc Lung Dis 2010;14:1024-31. [PubMed]
- Iram S, Zeenat A, Hussain S, et al. Rapid diagnosis of tuberculosis using Xpert MTB/RIF assay - Report from a developing country. Pak J Med Sci 2015;31:105-10. [PubMed]
- Gu Y, Wang G, Dong W, et al. Xpert MTB/RIF and GenoType MTBDRplus assays for the rapid diagnosis of bone and joint tuberculosis. Int J Infect Dis 2015;36:27-30. [Crossref] [PubMed]
- Chen ST, Zhao LP, Dong WJ, et al. The Clinical Features and Bacteriological Characterizations of Bone and Joint Tuberculosis in China. Sci Rep 2015;5:11084. [Crossref] [PubMed]