Pulmonary Langerhans cell histiocytosis: analysis of 14 patients and literature review
Introduction
Langerhans cell histiocytosis (LCH) is an orphan histiocytic disease, which includes disorders of unknown origin with extensive variations in clinical presentation and outcome. It is characterized by infiltration of the involved tissues by large numbers of Langerhans cells (LCs), often organized into granulomas (1). LCH can occur in any age, but the exact incidence is still unknown. According to the number of organs involved (2,3), LCH can be classified into three groups by the Histiocyte Society, including single-system, low-risk multisystem, and multisystem with risk-organ involvement. The lung can be involved primarily or secondarily in any group. Pulmonary involvement in multisystemic patients is uncommon, yet might indicate poor prognosis (4). On the contrary, solitary pulmonary involvement is more common, so it is named as PLCH. PLCH differs from the pulmonary involvement in multisystem disease, which is recognized as the more common form now (3,5).
PLCH is a pulmonary interstitial disease, which can be asymptomatic or manifest with respiratory symptoms. The pathogenesis of PLCH is uncertain, previous studies found that PLCH had high incidence in smokers (1,6-13), particularly in young adult smokers (14), but precise epidemiological data was not available. The relative frequency in men and women is equal (8), which probably because of the increased prevalence of smoking among women in recent years (15). Among adults with PLCH, the long term survival is shorter than that of general population, and their health is substantially affected (14). Ideally, the diagnosis of PLCH must be confirmed by tissue biopsy, however, some patients were unwilling to perform this invasive test because of the risk as well as the cost. Hence, PLCH is easily misdiagnosed clinically. The purpose of this article was to emphasize the clinical features especially radiologic features of PLCH patients; furthermore, the current literature was also reviewed.
Methods
This study retrospectively analyzed the clinical data of 14 patients with PLCH. All the coherent patients were hospitalized at the Shanghai Pulmonary Hospital, Shanghai, China, between December 2008 and June 2012. The diagnosis of PLCH should fulfill one of the following criteria: (I) disease proven by lung biopsy; (II) a positive biopsy of an extra thoracic localization of the disease or the presence of diabetes insipidus associated with characteristic lung HRCT findings; or (III) the combination of an appropriate clinical setting, a typical lung HRCT pattern (showing both nodules and cysts) and the exclusion of alternative diagnoses (16).
The following clinical data were obtained from the medical records: age, sex, smoking history, clinical features and PFTs. All patients had routine peripheral blood and serum biochemical tests. The radiological tests included chest X-ray and CT scan. Six patients underwent surgical lung biopsy, one patient got CT-guide percutaneous lung puncture, and one patient underwent biopsy from enlarged cervical lymph nodes. The remaining six patients were diagnosed based on the clinical-radiological data. The study protocol was approved by the Ethics Committee of Shanghai Pulmonary Hospital (approval number: K16-265). All participants were given informed consent before taking part.
Results
The age of patients (11 males and 3 females) ranged from 21 to 72 years, and the average age was 42.79 (±13.71) years old. All the male patients as well as one female patient had a long smoking history (Table 1). The symptoms of PLCH patients included cough (in nine patients), exertional dyspnea (in seven patients), expectoration (in three patients), chest tightness and chest pain (in one patient, separately). Three asymptomatic patients were found having lung shadows by radiological examination. Spontaneous pneumothorax was found in three patients. Neither hemoptysis nor extra-pulmonary symptom was detected in 14 PLCH patients (Table 2).
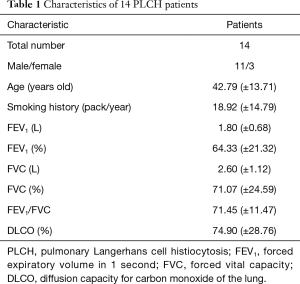
Full table
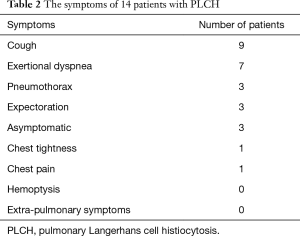
Full table
Only six patients got PFTs data, FEV1 ranged from 1.03 to 2.68 L, FVC ranged from 1.09 to 4.21 L, and FEV1/FVC ranged from 62.73% to 93.57%. Among the four tested patients, DLCO% ranged from 32.5 to 96.5. No distinct abnormalities and significant differences were found in blood routine examination and serum biochemical tests.
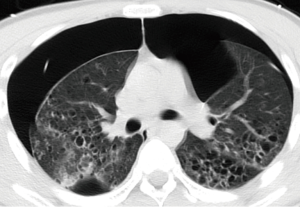
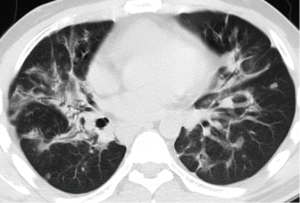
There were various appearances including nodular, cystic, patch and cords shadows revealed by chest X-ray or CT examination. The nodular shadows were found in seven patients, cystic shadows in six patients, patchy shadows in three patients and cord shadows in two patients. No specific distribution was found for the lung shadows. Other abnormities on CT scan included pleural thickening (in two patients), pleural effusion (in one patient) and mediastinal lymph node enlargement (in three patients). All the CT scan observations are given in Table 3, and typical images can be seen in Figures 1,2.
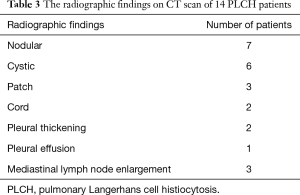
Full table
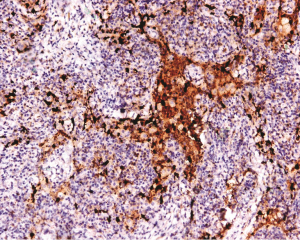
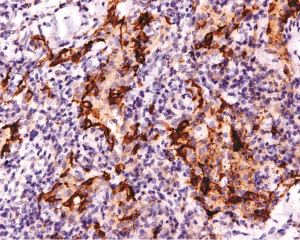
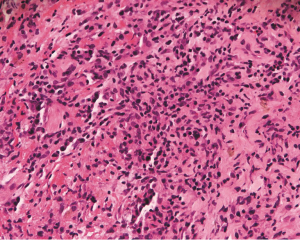
The LC lesions were discovered through histological examination in biopsied tissue. LCs were positive for S-100 and CD1a by immunohistochemical staining. Imaging from biopsies can be seen in Figures 3-5.
The total of 12 smokers were recommended to quit smoking, 2 out of 3 patients with recurrent pneumothorax were treated by closed thoracic drainage, and 7 patients received symptomatic relief and supportive treatment. Eleven patients followed up at outpatient department or by telephone interview. Four patients felt improved symptoms with smoking cessation, while 2 felt not. Two patients with recurrent pneumothorax underwent pleura fixation because of thoracic drainage failure. Three patients died within three years after discharge, the cause of death was pulmonary failure in two patients and unknown in the rest.
Discussion
The present study aims to retrospectively analyze the clinical data of 14 patients with PLCH as well as to review the relevant literature. So far, we still know little about the clinical features of PLCH despite several studies have been reported (8,17,18). We hope our study will add more data to PLCH, therefore, facilitate the diagnosis and management.
The pathogenesis of PLCH is largely unknown at present, although previous studies found that PLCH had higher incidence in smokers particularly in adults with smoking history (1,6-13). Several explanations have been brought up regarding how cigarette smoking potentially facilitate the formation of Langerhans’ cell nodules in the lungs (7,12). However, only small proportion of smokers develops PLCH, necessitating the correlation between smoking and PLCH to be further investigated.
The presentation of PLCH can be various (15,19,20), in spite of diffuse lung involvement, symptoms can be presented as insidious onset or nonspecific manifestations. About 25% of cases were asymptomatic, respiratory symptoms mainly including dry cough and dyspnoea on exertion were in approximately two-thirds of cases, and spontaneous pneumothorax was in 10–20% of cases (1). This data is approximate to our study. However, symptoms such as cough and exertional dyspnea are so common that each of them can be seen in other respiratory diseases, and this leads to the difficulty of diagnosing PLCH. The occurrence of pneumothorax seems more common in PLCH patients, with 60% of such patients experiencing at least one episode (21-24). PLCH seemed to associate with patients experiencing recurrent pneumothorax (25). In fact, sudden death due to bilateral pneumothorax of PLCH patient were reported (26). Hence, recurrent pneumothorax should be paid attention to not only because of the association with PLCH but also its life-threatening potential. In our current study, all PLCH patients presented as single-system symptoms without extrapulmonary manifestations.
At early phase of PLCH patients, due to pulmonary vascular lesion and ventilation limitation, pulmonary function may present in the pattern of restrictive ventilation. With the progression of disease, obstructive ventilation dysfunction could evolve. Pulmonary function can present as normal, obstructive or restrictive. Early phases present nodular lesions, while more advanced phases are appeared by cysts and fibrotic changes (27). In this study, 1 patient had normal pulmonary function and 2 had restrictive ventilation dysfunction, which were consistent to the early phase, while 1 patient had obstructive ventilation dysfunction and 2 had mixed ventilation dysfunction, which were also consistent to their advanced disease phase. Three patients showed DLCO% >80%, which are at the early stages of PLCH confirmed by CT scan; the decrease of DLCO is not evident, possibly because the sample size is small. Previous study found that lung function deteriorated in 60% of the patients and improved in 20% (16). Serial lung function assessment is necessary for the follow-up of PLCH patients to identify their disease progression (28). However, in the current study, PFTs data during follow-up are not achieved, so a larger follow-up study is needed to prove the findings in the previous study (16).
The radiological manifestations of PLCH were reported to be cystic and nodular shadows (29), and located in the middle or upper segments of the lung (30), which differed from our study that the distribution of shadows was of no specificity. High resolution chest CT (HRCT) is a noninvasive examination that is very helpful in the etiological diagnosis of interstitial lung disease (31,32). In fact, history of smoking in combination with typical radiographic findings is key to suspect PLCH (27). Also, the characteristic CT features associated with demographic and clinical factors could be diagnostic for PLCH and might spare lung biopsy (33). At early phase of PLCH, HRCT shows 1–10 mm centrilobular nodules that may be cavitary, as disease evolves, cystic lesions predominate over nodules (5,33). Previous study found that nodular abnormalities revealed by CT scans correspond to granulomatous lesions in lung tissue, and are suggestive of an active process (34), meanwhile, thin-wall cysts on HRCT often correspond to cavitary and inflammatory granulomas, suggesting that in PLCH patients, if lesions present in a cystic scan pattern, the persistence of an active, while limited granulomatous process cannot be excluded (34,35). Another study found that cystic lesions suggested a reversible disease, and to patients who had cystic lesions, early intervention was necessary (36). Pleural effusion is unusual radiographic findings of PLCH, which has not been reported in recent literatures. In the present study, two patients were found having pleural thickening and one patient pleural effusion, and pleural effusion might be correlated with the recurrent pneumothorax. Although enlargement of hilar/mediastinal lymph nodes is generally concerning for malignancy, no evidence was found to prove malignancy among all three patients who had mediastinal/hilar lymphadenopathy.
Definitive diagnosis of PLCH depends on tissue biopsy through invasive procedure (24). The open lung biopsy or thoracoscope is the main approach for the diagnosis of PLCH (37). The histopathologic features of PLCH are granulomatous nodules composed of LCs, eosinophils, and scattered other chronic inflammatory cells, and LCs are with grooved nuclear membrane and eosinophilic cytoplasm (15). A key morphological feature to distinguish LCH cells from other cells is their highly convoluted nuclear membranes (38). Immunohistochemical stains will greatly facilitate the discovery of LCs which are positive for CD1a and S-100 (39). In addition, the rod-like Birbeck particles could be seen in cytoplasm by using electron microscopy (39).
However, Hagmeyer et al. (40) thought that HRCT should be used for differential diagnosis and lung biopsy was often unnecessary in smoking-related interstitial lung diseases (embrace PLCH). Also, previous study found that a lung CT at diagnosis of PLCH was informative (16) and confident (31,41). In the present study, not all the patients underwent tissue biopsy by open lung biopsy or thoracoscope, and this is a limitation of our study. Some patients decided not to perform this invasive test after serious consideration, because of the high risk as well as the expensive cost. For those patients, radiological examination as an invasive method may play crucial roles. In the future, more prospective studies with large sample size should be performed to pay close attention to clinical characteristics of PLCH, such that the chance of correct diagnosis can be improved.
In conclusion, PLCH is still an orphan disease and maybe related to smoking. Clinical symptoms such as cough and exertional dyspnea seem to be non-specific. Recurrent pneumothorax should be paid attention to due to its potential link to PLCH. The characteristic radiological manifestation is cystic or nodular shadow, which plays crucial roles in diagnosing PLCH.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tazi A. Adult pulmonary Langerhans' cell histiocytosis. Eur Respir J 2006;27:1272-85. [Crossref] [PubMed]
- Histiocytosis syndromes in children. Writing Group of the Histiocyte Society. Lancet 1987;1:208-9. [PubMed]
- Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol 1997;29:157-66. [Crossref] [PubMed]
- Aricò M, Egeler RM. Clinical aspects of Langerhans cell histiocytosis. Hematol Oncol Clin North Am 1998;12:247-58. [Crossref] [PubMed]
- Abbott GF, Rosado-de-Christenson ML, Franks TJ, et al. From the archives of the AFIP: pulmonary Langerhans cell histiocytosis. Radiographics 2004;24:821-41. [Crossref] [PubMed]
- Margaritopoulos GA, Vasarmidi E, Jacob J, et al. Smoking and interstitial lung diseases. Eur Respir Rev 2015;24:428-35. [Crossref] [PubMed]
- Crotty Alexander LE, Shin S, Hwang JH. Inflammatory Diseases of the Lung Induced by Conventional Cigarette Smoke: A Review. Chest 2015;148:1307-22. [Crossref] [PubMed]
- Elia D, Torre O, Cassandro R, et al. Pulmonary Langerhans cell histiocytosis: a comprehensive analysis of 40 patients and literature review. Eur J Intern Med 2015;26:351-6. [Crossref] [PubMed]
- Neurohr C, Behr J. Changes in the current classification of IIP: A critical review. Respirology 2015;20:699-704. [Crossref] [PubMed]
- Walsh SL, Nair A, Desai SR. Interstitial lung disease related to smoking: imaging considerations. Curr Opin Pulm Med 2015;21:407-16. [Crossref] [PubMed]
- Cheng S, Mohammed TL. Diffuse smoking-related lung disease: emphysema and interstitial lung disease. Semin Roentgenol 2015;50:16-22. [Crossref] [PubMed]
- Gupta N, Vassallo R, Wikenheiser-Brokamp KA, et al. Diffuse Cystic Lung Disease. Part I. Am J Respir Crit Care Med 2015;191:1354-66. [Crossref] [PubMed]
- Margaritopoulos GA, Harari S, Caminati A, et al. Smoking-related idiopathic interstitial pneumonia: A review. Respirology 2016;21:57-64. [Crossref] [PubMed]
- Vassallo R, Ryu JH, Schroeder DR, et al. Clinical outcomes of pulmonary Langerhans'-cell histiocytosis in adults. N Engl J Med 2002;346:484-90. [Crossref] [PubMed]
- Vassallo R, Ryu JH, Colby TV, et al. Pulmonary Langerhans'-cell histiocytosis. N Engl J Med 2000;342:1969-78. [Crossref] [PubMed]
- Tazi A, Marc K, Dominique S, et al. Serial computed tomography and lung function testing in pulmonary Langerhans' cell histiocytosis. Eur Respir J 2012;40:905-12. [Crossref] [PubMed]
- Aydoğdu K, Günay E, Fındık G, et al. Pulmonary Langerhans cell histiocytosis; characteristics of 11 cases. Tuberk Toraks 2013;61:333-41. [Crossref] [PubMed]
- Schönfeld N, Dirks K, Costabel U, et al. A prospective clinical multicentre study on adult pulmonary Langerhans' cell histiocytosis. Sarcoidosis Vasc Diffuse Lung Dis 2012;29:132-8. [PubMed]
- Tazi A, Soler P, Hance AJ. Adult pulmonary Langerhans' cell histiocytosis. Thorax 2000;55:405-16. [Crossref] [PubMed]
- Sundar KM, Gosselin MV, Chung HL, et al. Pulmonary Langerhans cell histiocytosis: emerging concepts in pathobiology, radiology, and clinical evolution of disease. Chest 2003;123:1673-83. [Crossref] [PubMed]
- Didilis VN, Bitzikas GI, Mikroulis DA, et al. Pulmonary invasion in multisystem Langerhans cell histiocytosis. Ann Thorac Surg 2003;75:1656. [Crossref] [PubMed]
- Mendez JL, Nadrous HF, Vassallo R, et al. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest 2004;125:1028-32. [Crossref] [PubMed]
- Badra FA, Karamouzis MV, Zolota V, et al. A case of multiorgan Langerhans' cell histiocytosis presented with pneumothorax. Eur J Intern Med 2004;15:467-9. [Crossref] [PubMed]
- Lin MW, Chang YL, Lee YC, et al. Pulmonary Langerhans cell histiocytosis. Lung 2009;187:261-2. [Crossref] [PubMed]
- Muramatsu T, Shimamura M, Furuichi M, et al. Pulmonary langerhans cell histiocytosis with recurrent pneumothorax. Ann Thorac Surg 2011;91:e83-4. [Crossref] [PubMed]
- Nakhla H, Jumbelic MI. Sudden death of a patient with pulmonary Langerhans cell histiocytosis. Arch Pathol Lab Med 2005;129:798-9. [PubMed]
- Castoldi MC, Verrioli A, De Juli E, et al. Pulmonary Langerhans cell histiocytosis: the many faces of presentation at initial CT scan. Insights Imaging 2014;5:483-92. [Crossref] [PubMed]
- Tazi A, de Margerie C, Naccache JM, et al. The natural history of adult pulmonary Langerhans cell histiocytosis: a prospective multicentre study. Orphanet J Rare Dis 2015;10:30. [Crossref] [PubMed]
- Greiwe AC, Miller K, Farver C, et al. AIRP best cases in radiologic-pathologic correlation: Pulmonary langerhans cell histiocytosis. Radiographics 2012;32:987-90. [Crossref] [PubMed]
- Bonelli FS, Hartman TE, Swensen SJ, et al. Accuracy of high-resolution CT in diagnosing lung diseases. AJR Am J Roentgenol 1998;170:1507-12. [Crossref] [PubMed]
- Grenier P, Valeyre D, Cluzel P, et al. Chronic diffuse interstitial lung disease: diagnostic value of chest radiography and high-resolution CT. Radiology 1991;179:123-32. [Crossref] [PubMed]
- Grenier P, Chevret S, Beigelman C, et al. Chronic diffuse infiltrative lung disease: determination of the diagnostic value of clinical data, chest radiography, and CT and Bayesian analysis. Radiology 1994;191:383-90. [Crossref] [PubMed]
- Ahuja J, Kanne JP, Meyer CA, et al. Histiocytic disorders of the chest: imaging findings. Radiographics 2015;35:357-70. [Crossref] [PubMed]
- Brauner MW, Grenier P, Tijani K, et al. Pulmonary Langerhans cell histiocytosis: evolution of lesions on CT scans. Radiology 1997;204:497-502. [Crossref] [PubMed]
- Soler P, Bergeron A, Kambouchner M, et al. Is high-resolution computed tomography a reliable tool to predict the histopathological activity of pulmonary Langerhans cell histiocytosis? Am J Respir Crit Care Med 2000;162:264-70. [Crossref] [PubMed]
- Kim HJ, Lee KS, Johkoh T, et al. Pulmonary Langerhans cell histiocytosis in adults: high-resolution CT-pathology comparisons and evolutional changes at CT. Eur Radiol 2011;21:1406-15. [Crossref] [PubMed]
- Suri HS, Yi ES, Nowakowski GS, et al. Pulmonary langerhans cell histiocytosis. Orphanet J Rare Dis 2012;7:16. [Crossref] [PubMed]
- Juvet SC, Hwang D, Downey GP. Rare lung diseases III: pulmonary Langerhans' cell histiocytosis. Can Respir J 2010;17:e55-62. [Crossref] [PubMed]
- Gadner H, Minkov M, Grois N, et al. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood 2013;121:5006-14. [Crossref] [PubMed]
- Hagmeyer L, Randerath W. Smoking-related interstitial lung disease. Dtsch Arztebl Int 2015;112:43-50. [PubMed]
- Richards JC, Lynch DA, Chung JH. Cystic and nodular lung disease. Clin Chest Med 2015;36:299-312. ix. [Crossref] [PubMed]