Investigating CTCs in NSCLC—a reaction to the study of Jia-Wei Wan: a preliminary study on the relationship between circulating tumor cells count and clinical features in patients with non-small cell lung cancer
Liquid biopsies are emerging as a patient-friendly approach for estimating biomarkers to predict treatment outcome and overall survival. Detection and characterization of circulating tumor cells (CTCs) is a promising biomarker and its application for non-small cell lung cancer (NSCLC) is currently under investigation. The FDA approved CellSearch system is used as the standard test. The most challenging hurdle in this and other techniques is to discern the CTCs from the background noise of normal blood cells. In the CellSearch system, lysis of erythrocytes, separating leukocytes with anti-CD antibodies, and immunomagnetic enrichment of cancer cells from blood samples expressing membranous epithelial cell adhesion molecule (EpCAM) protein is used. The relevant detection rate using CellSearch for prognosis is set by the developer on >2 (for metastatic colorectal cancer) or >4 CTCs (for metastatic breast and prostate cancer) per 7.5 mL blood sample. Clinical use of the CellSearch system is currently limited in NSCLC because it fails to detect CTCs at an acceptable rate and at a sufficient high yield. Extrapolation of CTC frequency distribution in 7.5 mL of blood from patients with metastatic breast, colon and prostate cancer showed that probably all these patients had CTCs in circulation, but the sample volume was not sufficient to detect them in all patients. Therefore, novel methods for the detection of CTCs are being studied.
Methods of isolation
The different methods of isolation and detection of CTCs are based on the physical and biological properties of CTCs when compared to the normal cells. Generally these can be divided into three main groups: (I) measurement of the expression of epithelial specific proteins, also called the protein based or immunocytometry strategy; (II) mRNA or DNA based techniques, where the expression or presence of certain genetic sequences is measured; (III) and finally the use of the distinctive physical characteristics of CTCs, e.g., their size. Each method has its own merits.
Next to the CellSearch system, a novel technique using expression of epithelial specific proteins, based on the increased expression of the folate receptor in cancer cells has been developed and was used by Wan et al., published in Annals of Translational Medicine in Dec. 2015 (1). The expression of the folate receptor in several carcinomas including NSCLC was earlier reported by Parker et al. in 2005 and again by Nunez et al. and O’Shannessy et al. in 2012 (2-4). There are four known folate receptors (α, β, γ and δ). The γ and δ receptors are mainly expressed on T-cells, while β-receptors are expressed in a subpopulation of activated macrophages. The α-receptor physiologically plays a role in retaining folate in the body. They are primarily expressed on the apical side of the epithelial cells, for example epithelial cells lining the bladder. Organs expressing this α-receptor are the salivary glands, kidneys, lungs, pancreas and breast tissue. The receptor is thus primarily expressed in locations where excretion occurs.
The up regulation of folate α-receptors on cancer cells, and its relative absence in physiological circumstances in the hematological compartment, makes it a possible target to determine whether cells qualify as CTCs or not. In December 2013 a method based on this distinction was described by Lou et al. and by Yu et al. (5,6). Following lysis of red blood cells and depletion of white blood cells using CD45 leukocyte depletion magnetic beads, they used a conjugated ligand specific of the folate α-receptor, thereby binding all folate α-receptors present. Abundant unbound conjugates are depleted by a washing procedure. The remaining bound conjugates thus represent all folate α-receptors present in that sample. As a synthesized oligonucleotide was connected to the conjugates, quantification of the folate α-receptors is possible using qPCR. The amount of folate α-receptors is then divided by a value of 7.5×10−18 M (derived from the amount of folate α-receptors on a cancer cell line, specifically KB cells), giving the amount of CTC units (CTU) per 3 mL.
Following a validation study using spiked cells, Lou et al. (5) studied 72 NSCLC patients as well as 20 patients with benign diseases and 24 healthy donors. CTU amounts were significantly different between the groups: healthy patients had a mean of 6.7 CTU (range, 3.0–11.4), benign disease patients a mean of 6.0 (range, 1.6–8.7) while NSCLC patients disease stage I-III had a mean of 11.9 (range, 3.8–25.7) and disease stage IV had a mean of 20.9 (range, 6.8–75.0). The authors hypothesized that the background in healthy cases could have been attributed to indiscriminative binding of the oligonucleotides to the residual cells in the enriched samples.
Using a cut off of 8.5 CTU per 3 mL, they found a sensitivity of 82% and a specificity of 93% to detect CTC. Interestingly, they observed no difference between adenocarcinoma and squamous carcinoma in the amount of CTCs.
Yu et al. (6) tested the folate technique on 153 patients with NSCLC, 49 healthy controls and 64 patients with benign diseases. Using a threshold of 8.64 CTU per 3 mLblood, the method showed a sensitivity of 73% and a specificity of 84%. They did not find a significant difference in CTUs between the different disease stages.
Recently Chen et al. (7) investigated 473 patients with NSCLC; 227 patients with benign lung disease and 56 healthy donors. These were split in two groups, one group (236 NSCLC, 113 benign and 28 controls) was used to train a classifier while the second group (237 NSCLC, 114 benign and 28 controls) was used as a validation set of the model obtained from the training set.
The optimal cut off was determined to be 8.93 CTU per 3 mL, giving a sensitivity of 76% and a specificity of 82% for the validation set. They also related the CTUs to clinical stage, with a significant difference between stage I/II and III/IV, but not between stage III and IV. No difference in CTU count was found between the different histological subtypes as reported by Lou (5) earlier.
Finally, Wan et al. (1) used the same technique in 50 patients with both adenocarcinoma and squamous carcinoma from the lung. Nine patients had stage I disease, 11 stage II, 7 stage III and 23 had stage IV disease. As a control group, 35 patients with benign lung diseases and 28 healthy subjects were included. A clear difference in the CTUs per mL was observed between the NSCLC group and both the benign patients and the healthy controls (41.01, 1.03, and 0.34 CTU per mL respectively). In addition, higher CTU counts predicted a higher disease stage.
This technique using the folate α-receptors is fully dependent on the assumption that there are no other cells expressing these receptors, and that the tumor cells maintain their expression while in circulation as CTC. It is known that different processes such as epithelial-mesenchymal transition (EMT) may interfere with the expression of surface proteins; however whether this is also true for the folate α-receptor expression is unknown. Moreover, not all NSCLC subtypes or cells exhibit this protein and might therefore be missed using this method (2,3).
Also, in contrast to CTC determination in blood when looking at the folate α receptor expression, in a histological setting (biopsies) there seems to be a difference in expression between adenocarcinoma (higher) and squamous carcinoma lung cancer patients (2,3).
Of interest is the ‘background noise’ present in healthy controls. This background can be caused by cross binding with the β-receptor, presented by remaining leukocytes. Another possibility is the non-specific binding of the oligonucleotide by different normal cells, for example macrophages or red blood cells that remained behind in the enriched sample, or the presence of a small subpopulation of activated macrophages that does present the α receptor (5,6).
Finally, this new method has not yet been correlated to survival. Hopefully this will be the next step.
In line with other results
CTCs have already shown to be prognostic in both lung and other solid tumors over the last decade. High CTC counts are detrimental in all solid tumor types. The change of CTC count has been linked to better survival and to response therapy (8-11). However, there are many different methods to obtain the CTCs that are used and cut off values differ substantially from 1 to 8 CTCs per 7.5 mL blood (9,12-16) (Table 1).
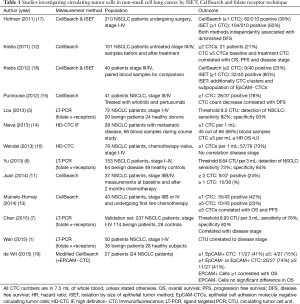
Full table
Currently the single FDA approved method (for colon, prostate and metastatic breast cancer, but not lung carcinoma) is the CellSearch system. The CellSearch system uses the aforementioned immunocytometric methods to discriminate CTCs. After separating the plasma from the solid blood components, magnetic beads aimed at the EpCAM are used to magnetically separate CTCs from the other cells. Followed by staining using monoclonal antibodies against cytokeratin and CD45 in combination with the nuclear cell staining 4',6-diamidino-2-phenylindole (DAPI). EpCAM and DAPI positive cells that are CD45 negative are considered to be CTCs. This validated technique is highly reproducible and has clear prognostic value in many different cancer subtypes, indicating that a clinically relevant portion of cells are identified. As this system focuses on EpCAM positive cells all cells not exhibiting this molecule are therefore not recognized as tumor cells. While it is known that tumor cells do not always express EpCAM (20,21), the prognostic impact of these EpCAM negative (EpCAM-) cells was negligible when adding a micro sieve to the collection of the blood discarded by the CellSearch after immunomagnetic enrichment of EpCAM + CTCs (19). Still, more information is necessary before a definitive conclusion can be drawn.
A second problem is that the CellSearch system only isolates CTCs in a small fraction of patients per 7.5 mL blood, even in the case of metastasized disease (12).
The mRNA based techniques are generally PCR-based in a single or multi marker approach. These techniques are difficult to reproduce and are unable to assess cell numbers. Additionally, markers like TTF-1 or CEA detected by reverse transcriptase PCR are not necessarily derived from CTCs. Other techniques using DNA from circulating cells are only useful against the specified target, such as the epidermal growth factor receptor (EGFR) mutations (22).
Finally, measurements based on specific properties of CTCs, mostly filtering methods, have the advantage of capturing individual cells, making it possible to study the vital cells after isolation. Using this method a relative larger number of CTCs are found in 7.5 mL of blood (17,18). For example, the ISET method by RareCell has a reported sensitivity of up to 1 cell per mL whole blood (18). This method operates by filtering cells through a membrane. Cells larger than 8 microns remain on the membrane and are transferred into wells where they can be studies using a microscope. The ISET method identifies CTCs in a larger percentage of patients and in greater numbers: Specifically Krebs et al. (18) identified CTCs in 32 out of 40 NSCLC patients (80%) using the ISET method with a mean number of cells per patient of 71 (range, 0–1,045). The CellSearch method isolated cells in 9 out of 40 patients (23%) with a mean of 4 cells per patient (range, of 0–78 cells) (18). Using the ISET method they also identified EpCAM- CTCs and detected CTC clusters, which were not found when the CellSearch system was used. Although they did not use the modified CellSearch system described by De Wit et al. (19). The clinical relevance of these EpCAM- cells is still largely unknown and needs further studying as mentioned before.
Hurdles to overcome and future prospects
The most important hurdle is the identification of a single or few CTCs amongst millions of white blood cells. It is necessary to increase the yield if we want to make CTCs available for other diagnostic techniques, such as single-cell whole genome sequencing or strand specific sequencing (23,24) which identifies both copy number alterations and translocation breakpoints. One way to increase the yield of CTCs is to filter more whole blood by using diagnostic leukapheresis (DLA) (25). DLA is a standard clinical method to isolate mononuclear cells (MNCs) from blood. It is currently used as routine practice in hematological diseases. It improves the detection rate of CTCs to 56% for all stages of lung cancer. Currently only few centers are exploring this approach. Another possibility is the use of filters such as the ISET technique, but other filters and markers are constantly being developed, causing the field to change continuously. More research still needs to be performed for developing new techniques and comparing all methods, but CTCs have the possibility to play a major role in the future for prognosis, tumor typing and selection and follow up of therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wan JW, Gao MZ, Hu RJ, et al. A preliminary study on the relationship between circulating tumor cells count and clinical features in patients with non-small cell lung cancer. Ann Transl Med 2015;3:352. [PubMed]
- Nunez MI, Behrens C, Woods DM, et al. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFRmutation. J Thorac Oncol 2012;7:833-40. [Crossref] [PubMed]
- O’Shannessy DJ, Yu G, Smale R, et al. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget 2012;3:414-25. [Crossref] [PubMed]
- Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 2005;338:284-93. [Crossref] [PubMed]
- Lou J, Ben S, Yang G, et al. Quantification of rare circulating tumor cells in non-small cell lung cancer by ligand-targeted PCR. PLoS One 2013;8:e80458. [Crossref] [PubMed]
- Yu Y, Chen Z, Dong J, et al. Folate receptor-positive circulating tumor ells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol 2013;6:697-702. [Crossref] [PubMed]
- Chen X, Zhou F, Li X, et al. Folate Receptor–Positive Circulating Tumor Cell Detected by LT-PCR–Based Method as a Diagnostic Biomarker for Non–Small-Cell Lung Cancer. J Thorac Oncol 2015;10:1163-71. [Crossref] [PubMed]
- Breitenbuecher F, Hoffarth S, Worm K, et al. Development of a Highly Sensitive and Specific Method for Detection of Circulating Tumor Cells Harboring Somatic Mutations in Non-Small-Cell Lung Cancer Patients. PLoS ONE 2014;9:e85350. [Crossref] [PubMed]
- Dorsey JF, Kao GD, Macarthur KM, et al. Tracking Viable Circulating Tumor Cells (CTCs) in the Peripheral Blood of Non-Small Cell Lung Cancer Patients Undergoing Definitive Radiation Therapy: Pilot Study Results. Cancer 2015;121:139-49. [Crossref] [PubMed]
- Hiltermann TJ, Pore MM, Van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: A predictive and prognostic factor. Ann Oncol 2012;23:2937-42. [Crossref] [PubMed]
- Juan O, Vidal J, Gisbert R, et al. Prognostic significance of circulating tumor cells in advanced non-small cell lung cancer patients treated with docetaxel and gemcitabine. Clin Transl Oncol 2014;16:637-43. [Crossref] [PubMed]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and Prognostic Significance of Circulating Tumor Cells in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 2011;29:1556-63. [Crossref] [PubMed]
- Muinelo-Romay L, Vieito M, Abalo A, et al. Evaluation of circulating tumor cells and related events as prognostic factors and surrogate biomarkers in advanced NSCLC patients receiving first-line systemic treatment. Cancers (Basel) 2014;6:153-65. [Crossref] [PubMed]
- Nieva J, Wendel M, Luttgen M. High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis. Phys Biol 2012;9:016004. [Crossref] [PubMed]
- Punnoose EA, Atwal S, Liu W, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012;18:2391-401. [Crossref] [PubMed]
- Wendel M, Bazhenova L, Boshuizen R, et al. Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology. Phys Biol 2012;9:016005. [Crossref] [PubMed]
- Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch AssayTM and the isolation by size of epithelial tumor cell method. Int J Cancer 2011;129:1651-60. [Crossref] [PubMed]
- Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012;7:306-15. [Crossref] [PubMed]
- de Wit S, van Dalum G, Lenferink AT, et al. The detection of EpCAM(+) and EpCAM(-) circulating tumor cells. Sci Rep 2015;5:12270. [Crossref] [PubMed]
- Kim Y, Hyo Song K, Zheng YC, et al. Clinicopathological implications of EpCAM expression in adenocarcinoma of the lung. Anticancer Res 2009;29:1817-22. [PubMed]
- Lecharpentier A, Vielh P, Perez-Moreno P, et al. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer 2011;105:1338-41. [Crossref] [PubMed]
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of Mutations in EGFR in circulating Lung-Cancer cells. New Eng J Med 2008;359:366-77. [Crossref] [PubMed]
- Falconer E, Lansdorp PM. Strand-seq: a unifying tool for studies of chromosome segregation. Semin Cell Dev Biol 2013;24:643-52. [Crossref] [PubMed]
- Ni X, Zhuo M, Su Z, et al. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc Natl Acad Sci 2013;110:21083-8. [Crossref] [PubMed]
- Fischer JC, Niederacher D, Topp SA, et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc Natl Acad Sci U S A 2013;110:16580-5. [Crossref] [PubMed]


