For non-small cell lung cancer with T3 (central) disease, sleeve lobectomy or pneumonectomy?
Introduction
Lung cancer is the most commonly diagnosed cancer as well as the leading cause of cancer death in males worldwide (1). Surgery for patients with non-small cell lung cancer (NSCLC) involving proximal bronchi can be challenging. Pneumonectomy (PN) is the most extensive pulmonary resection to ensure complete resection for these patients. However, PN is associated with high rates of complications, especially for patients with compromised pulmonary function. Sleeve lobectomies were introduced as a new method that may facilitate to operate more patients (patients who do not tolerate PN). At the same time patients that used to undergo PN are diverted to parenchyma sparing surgery (2,3). In 1952, Allison performed the first successful right upper lobe sleeve lobectomy (SL) for a patient with bronchogenic carcinoma (4). After comparison with PN, SL has become an accepted and preferred procedure for its similar or better long-term survival as well as lower risk of operative mortality and higher quality of life, regardless of pulmonary function (5-13).
However, It is recognized that PN patients likely have more advanced disease (4-6,8,10,12-14). In order to minimize this selection bias between the two groups, exact same T stage should be the selection criteria. Any T stage can be analyzed, however, T3 central (tumor in the main bronchus <2 cm distal to the carina or atelectasis/obstructive pneumonitis of entire lung) has more value than any others to evaluate the oncologic efficacy of SL. The explanation is that the extent of T3 central disease in the SL group is getting close to PN group. In addition, it is technically more challenging due to high risk of positive bronchial margin for T3 (central) compared with T1 and T2. For T4 disease, it is poor candidate for SL (15). The presence of N2 significantly impairs long-term outcomes among sleeve lobectomies due to systemic recurrences (16-18). Furthermore, there were no difference in survival with N0 and N1 disease (17-20). Comparison between parenchyma-sparing SL and PN for central T3 NSCLC with absence of N2 disease is the less discussed previously. The result of this study is worthy for surgeon’s future decision making regardless of pulmonary function.
Methods
Patient data
This retrospective cohort study used an electronic database of consecutive patients between April 1998 and October 2012. This study was approved by the China-Japan Friendship Hospital Ethics Committee. The identify number was 2015-XWK-21. The patients in this study signed the informed consent before enrollment. Different NSCLC tumor node metastasis (TNM) stage editions existed during the last 14 years. One pathologists have reviewed all of the pathological reports and use the standard of Union for International Cancer Control, WHO, 7th edition, 2009. Seventeen patients’ pathological stage has been adjusted. Patients who underwent R0 resection (margin-negative) with SL or PN of T3 (central) N0-1 M0 NSCLC, without induction chemotherapy or induction radiotherapy, were eligible.
Evaluation
Preoperative workup included clinical history, physical examination, and chest computed tomography (CT) scan with intravenous contrast no more than 1 month before resection, pulmonary function test, blood gas analysis, cardiac evaluation, bronchoscopy and basic examinations as usual. Abdominal B-ultrasound, cerebral magnetic resonance imaging (MRI) and isotopic bone scanning were examinations to exclude metastatic disease. Cervical mediastinoscopy, FDG-PET (fluorodeoxyglucose-position emission tomographies), or EBUS-TBNA (endobronchial ultrasound-guided transbronchial needle aspiration) were employed to exclude N2 disease.
All SL patients were assessed preoperatively, and their pulmonary function tests were deemed adequate to tolerate potential PN [forced expiratory volume in 1 second (FEV1) and diffusing capacity of the lung for carbon monoxide >60% predicted]. All bronchoscopic, operative and pathology reports were reviewed in details to confirm the tumor node metastasis (TNM) stage.
Techniques of bronchoplasty
The choice of operative techniques varied slightly according to the surgeons’ preferences during the study period, but generally was as follows: lobe mobilization began with dissection of pulmonary artery, identification of its lobar broaches, and followed by division of corresponding pulmonary vein, incomplete fissures. After these hilar structures were transected, airway dissection and bronchial resection were performed. Bronchial margins were confirmed tumor-free by frozen histology evaluation. End-to-end bronchial anastomosis was performed with interrupted suture. Absorbable 3-0 or 4-0 sutures (Vicryl, Ethicon, Inc, Somerville, NJ, USA) were used to decrease the rate of stricture and granuloma formation (21). The anastomoses were covered by mediastinal pleura, pericardial fat pads, or intercostal muscle flap. All patients underwent curative oncologic resection associated with complete mediastinal lymphadenectomy. At the end of surgery, bronchoscopy was performed to evaluate the anastomosis and clear the residual secretions from the airways prior to extubation.
Follow-up
Follow-up information was obtained from several sources, including telephone interviews with patients or their family, letters to the patients’ family, Social Security Death Index, or inquiry to oncologists and other physicians. There were nine patients unavailable for follow-up at the completion of study. The mean follow-up was 60 months (range, 2–254 months). Patients were followed postoperatively with bronchoscopy 1 and 3 months after operation to observe the healing of the anastomotic stoma and the eventual occurrence of fistula and stenosis, chest CT scan with contrast and blood tumor markers every six months for two years, then non-contrast-enhanced chest CT scan annually afterwards.
Loco regional recurrence was defined as recurrence of disease at the surgical resection margin area, bronchial stump and any recurrence in the ipsilateral hemithorax. Distant recurrences include the supraclavicular fossa, contralateral hilum and all other distant organs.
Statistical analysis
Clinical variables and outcomes were analyzed between sleeve lobectomies (SL) and pneumonectomies (PN). Data were collected and stored with an Excel database (Microsoft Corp, Redmond, Wash). Categorical variables were analyzed by means of χ2 test. Continuous variables were analyzed by Student t test. The start time of survival analysis was the date of pulmonary resection and the terminal event was death attributed to cancer or other causes, whereas patients alive were right-censored at the last available follow-up. Overall survival rates were calculated according to the method derived from the Kaplan-Meier method, and the differences between SL and PN were compared with the Log-rank test. Clinical and pathologic variables with a possible effect on survival were entered in a multivariate analysis (Cox proportional hazard model) to identify independent prognostic factors. The selected variables included age, histology type [squamous cell carcinoma (SCC) vs. non-squamous cell carcinoma (NSCC)], tumor stage (stage IIIA vs. stage IIB), tumor grade (poorly differentiated vs. well or moderately differentiated), and operative procedure (PN vs. SL). The statistical analysis was performed with the SPSS software, version 23.0. A P value less than 0.05 was considered to be significant.
Results
Between 1998 and 2014, 2,754 consecutive patients underwent curative lung resection for NSCLC. The number of all standard lobectomy and bilobectomy performed during the study period was 1913. During this period, 178 (6.5%) patients underwent SL and 294 (10.7%) underwent PN. Patients who had palliative surgery with positive margin (n=48) section were excluded from the study. A total of 100 patients (58 sleeve lobectomies and 42 pneumonectomies) received complete R0 resection of pT3 (central) N0-1 M0 NSCLC, Briefly, most patients were assigned to right upper lobe sleeve resections (n=38, 66%). Much fewer consisted of 6 (10%) left upper lobe sleeve resections and 2 (3%) left lower lobe resections. Remaining procedures were sleeve bilobectomy (n=12, 21%). Concomitant sleeve resection of the pulmonary artery was required in 7 patients (12.1%).
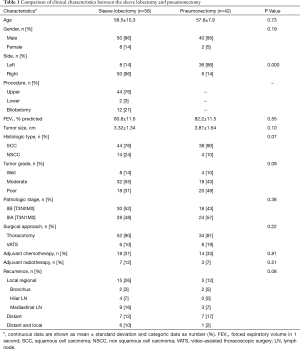
Patients were mostly male (90, 10 females), the mean age was 58.2±9.3 years (range, 26–75 years). The mean preoperative FEV1% was 81.3%±11.6%. The percentage of smoker was 78%. Thirty-seven patients had comorbidities (hypertension n=21; arrhythmia n=3; myocardial infarction n=2; chronic pulmonary disease n=8; peripheral vascular disease n=11; cerebrovascular disease n=2; diabetes n=7). Clinical characteristics, pathologic and adjuvant treatment details of the SL and PN are reported in Table 1. Age, pulmonary function, tumor size, and distribution of gender, histologic type, stages, and tumor differentiations were comparable between the two groups. The surgical approach, use of adjuvant chemotherapy and radiotherapy were not significantly different between two groups.
Full table
Operative mortality was defined as any death within 30 days of surgery or during the same hospital admission. The causes of postoperative mortalities were anastomotic dehiscence and bronchovascular fistula for SL. In PN group, one patient died from pneumonia on postoperative day five and one patient from acute respiratory distress syndrome (ARDS) on postoperative seven. Common complications after SL include 11 atrial arrhythmias, 8 sputum or blood clot retention and secondary atelectasis, and 7 prolonged air leak. Two patients had bronchial stricture. One patient was treated by bronchoscopic cryosurgery and the other received a stent to keep with patency of the bronchial lumen. Seven cases of atrial arrhythmias, 3 pneumonia and 3 vocal cord paralyses were most common events after PN.
During the follow-up period, recurrence occurred in 48% (28 of 58) of SL group (locoregional recurrence in 15, distant in 7, and loco regional-distant in 6 patients, respectively) and in 31% (13 of 42) of PN group (locoregional recurrence in 5, distant in 7, and loco regional-distant in 1 patient, respectively) (P=0.08). The sites of loco regional recurrences after SL were ipsilateral hilum in 4, distal mainstem bronchus in 2, and mediastinal lymph nodes in 9. For PN, 3 recurrences occurred in the mediastinal lymph nodes, and 2 occurred in the in the bronchial stump.
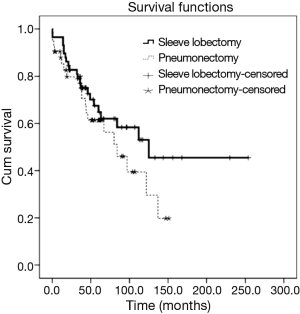
The 5-year overall survival was 63.4% for all patients. It was 64.8% [95% confidence interval (CI), 51.3% to 78.3%] for SL and 61.4% (95% CI, 45.3% to 77.5%) for PN, which was not significantly different (P=0.204, Figure 1). In univariate survival analysis, histologic of NSCC and stage IIIA predicted worse overall survival. In multivariable survival analysis that includes age, histologic type, stage, tumor grade, and operative procedure, there were no independent prognostic factors (Table 2).

Full table
Discussion
SL has evolved from an alternative to PN for patients with compromised lung function, to a standard procedure in selected patients with centrally located advanced NSCLC (4,5,22). Overall survival following SL for patients with NSCLC ranges from 39–53% at five years and 28–34% at ten years (4,5,12,16,18,19,23). SL is technically more demanding than PN, and the decision to select this procedure may be influenced by the surgeons’ experience. However, long-term results after SL have demonstrate that it can eradicate cancer to a degree similar to that of PN (5,6,12). And, SL can offer decreased postoperative mortality rates and comparable complication rates (4-6). Functional lung parenchyma are preserved after SL, so higher postoperative quality of life can be obtained with the benefit of reimplanted lobes (24-26). Furthermore, if a second primary lung cancer develops after SL, subsequent resection may be offered to select patients (27,28).
The mortalities following SL were anastomotic dehiscence and bronchovascular fistula in this study. The common cause was disruption of the bronchial blood flow, excessive tension at the anastomotic site, or inadequate protection of the anastomosis (29). Bronchial blood supply is located within the peribronchial tissue. Dissection should be carried out with consideration for the bronchial vessels, and unnecessary dissection should be avoided. The inferior pulmonary ligament is usually released at the beginning of the operation. In our experience and agree with others (22,29), hilar mobilization is also important to further minimize the tension at the anastomotic site by completely releasing pericardial tissue. In addition, when upper SL was performed, more attention should be paid to avoid unnecessary bronchial preservation. Because bronchus was more easily twisted after elevation of middle and/or lower lobe, resulting in increased anastomotic tension. The patient who died from bronchovascular fistula in this study received left upper lobe dual (broncho-vascular) sleeve resection. We reviewed the operation record and found neither bronchial nor pulmonary artery anastomosis was protected. Compared with the right side, both superior and posterior side of the left upper bronchus is surrounded by pulmonary artery. Due to this anatomical reason, bronchovascular fistula is more likely occur on the left side especially after dual sleeve resection. So both bronchial and pulmonary artery anastomoses should be covered by suitable tissues. We prefer intercostal muscle flap for the bronchial anastomosis and mediastinal pleura or pericardial fat pads for the pulmonary artery anastomosis. They can act as buffer between pulmonary artery and bronchus. Attention must be paid to the thickness of the buffer. Pulmonary artery should not be elevated too high to reduce the blood flow.
When NSCLC is present with T1-2 disease, SL is more easily to achieve adequate oncologic resection and associated with equal or better survival outcome than PN (6,7,9,16). Clinical studies for comparing the two procedures on T3 central disease were less conducted previously. One issue of sleeve resection if tumor invades the main bronchus less than 2 cm distal to the carina is the potentially increased rate of local recurrence. Although the difference of recurrences between SL and PN did not reach statistical significance, it seems that loco regional recurrences were more common after SL. The fact is that 2 of 15 (13%) patients with loco regional recurrences in the SL group were demonstrated that tumor came back around the site of bronchial anastomoses. Four were found with hilar positive lymph nodes, and nine were found with positive mediastinal lymph nodes. While, in the five loco regional recurrent patients treated by PN, 2 (40%) were found with bronchial stump recurrent. The other three recurrences occurred in the mediastinal lymph nodes. It was comparable if loco regional recurrence was referring to local bronchial recurrence [3% (2/58) vs. 5% (2/42)]. Furthermore, these nine lymph nodes recurrent patients in the sleeve resection group would not definitely benefit from PN. In this cohort of 100 patients limited to T3 (central) N0-1 M0 NSCLC treated with SL and PN, we found there were no significant differences between two groups considering operative mortality, morbidity, recurrence rates, and 5-year survival rates, only when a complete R0 resection via SL is achieved and documented.
In conclusion, our study support that SL does not compromise survival for NSCLC with T3 central disease compared with PN. It is an adequate oncologic resection and should be treated as the first line intervention for central lung cancer whenever technically feasible. Limitations are as follows: this is a retrospective, single-center study. Comparison of PN and SL is difficult. Although the same stage of pT3 (central) N0-1M0 was the selection criteria, patients in the two groups have different characters. Bias existed for procedure selection criteria by different experienced surgeons. It is hard to evaluate if surgeon could have done a sleeve he would have, and some patients require PN initially.
Acknowledgements
The authors thank Xiaofei Wang, Xin Meng, and Chunfang Zhang for their technical supports and cooperation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Wain JC. Bronchoplastic Resections. In: Kaiser LR. editor. Mastery of Cardiothoracic Surgery. Philadelphia: Lippincott-Raven, 1998:68-76.
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152-6; discussion 1156. [Crossref] [PubMed]
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Bagan P, Berna P, Pereira JC, et al. Sleeve lobectomy versus pneumonectomy: tumor characteristics and comparative analysis of feasibility and results. Ann Thorac Surg 2005;80:2046-50. [Crossref] [PubMed]
- Yoshino I, Yokoyama H, Yano T, et al. Comparison of the surgical results of lobectomy with bronchoplasty and pneumonectomy for lung cancer. J Surg Oncol 1997;64:32-5. [Crossref] [PubMed]
- Suen HC, Meyers BF, Guthrie T, et al. Favorable results after sleeve lobectomy or bronchoplasty for bronchial malignancies. Ann Thorac Surg 1999;67:1557-62. [Crossref] [PubMed]
- Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg 2000;119:814-9. [Crossref] [PubMed]
- Ghiribelli C, Voltolini L, Luzzi L, et al. Survival after bronchoplastic lobectomy for non small cell lung cancer compared with pneumonectomy according to nodal status. J Cardiovasc Surg (Torino) 2002;43:103-8. [PubMed]
- Martin-Ucar AE, Chaudhuri N, Edwards JG, et al. Can pneumonectomy for non-small cell lung cancer be avoided? An audit of parenchymal sparing lung surgery. Eur J Cardiothorac Surg 2002;21:601-5. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, Hasse J. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Takeda S, Maeda H, Koma M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: trends over time and 20-year institutional experience. Eur J Cardiothorac Surg 2006;29:276-80. [Crossref] [PubMed]
- Berry MF, Worni M, Wang X, et al. Sleeve lobectomy for non-small cell lung cancer with N1 nodal disease does not compromise survival. Ann Thorac Surg 2014;97:230-5. [Crossref] [PubMed]
- Faber LP. Sleeve lobectomy. Chest Surg Clin N Am 1995;5:233-51. [PubMed]
- Kim YT, Kang CH, Sung SW, et al. Local control of disease related to lymph node involvement in non-small cell lung cancer after sleeve lobectomy compared with pneumonectomy. Ann Thorac Surg 2005;79:1153-61; discussion 1153-61. [Crossref] [PubMed]
- Terzi A, Lonardoni A, Falezza G, et al. Sleeve lobectomy for non-small cell lung cancer and carcinoids: results in 160 cases. Eur J Cardiothorac Surg 2002;21:888-93. [Crossref] [PubMed]
- Yildizeli B, Fadel E, Mussot S, et al. Morbidity, mortality, and long-term survival after sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;31:95-102. [Crossref] [PubMed]
- Fadel E, Yildizeli B, Chapelier AR, et al. Sleeve lobectomy for bronchogenic cancers: factors affecting survival. Ann Thorac Surg 2002;74:851-8; discussion 858-9. [Crossref] [PubMed]
- Rea F, Marulli G, Schiavon M, et al. A quarter of a century experience with sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2008;34:488-92; discussion 492. [Crossref] [PubMed]
- Wright CD. Sleeve lobectomy in lung cancer. Semin Thorac Cardiovasc Surg 2006;18:92-5. [Crossref] [PubMed]
- Predina JD, Kunkala M, Aliperti LA, et al. Sleeve lobectomy: current indications and future directions. Ann Thorac Cardiovasc Surg 2010;16:310-8. [PubMed]
- Tronc F, Grégoire J, Rouleau J, et al. Long-term results of sleeve lobectomy for lung cancer. Eur J Cardiothorac Surg 2000;17:550-6. [Crossref] [PubMed]
- Gómez-Caro A, Garcia S, Reguart N, et al. Determining the appropriate sleeve lobectomy versus pneumonectomy ratio in central non-small cell lung cancer patients: an audit of an aggressive policy of pneumonectomy avoidance. Eur J Cardiothorac Surg 2011;39:352-9. [Crossref] [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. J Thorac Oncol 2008;3:604-8. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Operative approach for multiple primary lung carcinomas. J Thorac Cardiovasc Surg 1998;115:836-40. [Crossref] [PubMed]
- Van Schil PE, Brutel de la Rivière A, Knaepen PJ, et al. Second primary lung cancer after bronchial sleeve resection. Treatment and results in eleven patients. J Thorac Cardiovasc Surg 1992;104:1451-5. [PubMed]
- Tronc F, Gregoire J, Deslauriers J. Bronchoplasty. In: Patterson G, Cooper J, Deslauriers J, et al. editors. Pearson's Thoracic & Esophageal Surgery. 3rd ed. Philadelphia: Churchill Livingstone Elsevier, 2008:894-908.
- Venuta F, Diso D, Anile M, et al. Techniques of protection and revascularization of the bronchial anastomosis. J Thorac Dis 2016;8:S181-5. [PubMed]