Long term complications following 54 consecutive lung transplants
Introduction
Lung transplantation (LuTx) is an established treatment for chronic pulmonary failure and recently outcome has improved significantly (1-3). The rejection rates after LuTx have sharply declined during the past decade due to the introduction of more powerful immunosuppression (4-6). Nevertheless, acute and chronic rejections remain common complications (5,6) and bronchiolitis obliterans syndrome represents a common cause of progressive graft failure (7-9). Due to the chronic immunosuppression, many patients develop osteoporosis, hypertension, diabetes mellitus, hyperlipidemia and renal insufficiency; gastro-esophageal reflux disease is another common complication following LuTx (10-12). Additional complications that must be expected are pulmonary embolism, cardiovascular events and neuropsychiatric disorders (13). Infection remains one of the most frequent and most dangerous complications for the lung recipient during all post-transplant periods despite administration of prophylactic antimicrobial agents (14-20). Infection of the lung allograft is a particularly common event and rare and multi drug resistant organisms must be expected (21-28). Post transplant malignancies are frequently associated with viruses such as EBV and HPV with additional oncogenic viruses to be considered (29-33). The long term complications not only have an impact on survival but also worsen quality of life of lung recipients (10,34,35). The exact prevalence of many of the mentioned complication during long term follow up is unknown. Data on outcome of LuTx beyond 10 years are still rarely reported.
This article retrospectively analyzes the early and long-term complications in a series of 54 consecutive lung transplants.
Methods
Patients
Between January 1993 and December 2000, a total of 54 consecutive lung transplants (17 unilateral and 37 bilateral) in 51 patients were performed at the Innsbruck University Hospital. There were 26 female and 25 male individuals with a median age of 54 (range, 16–68) years. Underlying diseases were chronic obstructive pulmonary disease (n=23), cystic fibrosis (n=5), emphysema (n=7) and lung fibrosis (n=7), the remaining 12 patients were transplanted for various other pulmonary diseases.
Surgical technique and immunosuppression
Surgical techniques and perioperative management was carried out according to standard protocols (21). Prophylactic immunosuppression consisted of cyclosporine A (trough levels of 200–300 ng/dL) based triple drug therapy in the majority of patients. In 16 patients, tacrolimus (trough levels of 12–18 ng/dL) was used instead of CsA. Mycophenolic acid (2 g daily dose) was used following 27 transplants and azathioprine (1–2 mg/kg bodyweight) in another 27 cases. ATG induction (Fresenius, 3 mg/kg/d or Merieux, 1.5–2 mg/kg/d) was used in 29 patients; a total of 22 patients received an interleukin 2 receptor antagonist. Basiliximab (Simulect®, Novartis) was used in five patients at a dosage of 20 mg given twice and daclizumab (Zenapax®, Roche) was used in another 17 patients at a dosage of 1 mg/kg bodyweight (total seven doses). First and second rejection episodes were treated with high dose methylprednisolone (500 mg on three consecutive days) steroid resistant rejection was treated with ATG or OKT3. Maintenance trough levels for CsA were 50–150 ng/mL and for TAC 3–8 ng/mL.
Follow up
During first hospitalization, an intensive graft and infection monitoring was performed. After discharge, patients were examined after one, three, six and twelve months; afterwards in yearly intervals with additional visits whenever indicated. Patients underwent pulmonary function test, chest X-ray, arterial blood gas analysis and blood chemistry. Bronchoscopy with bronchoalveolar lavage or biopsy was performed at fixed intervals; biopsies were judged according to the ISHLT classification (36). In addition, specimens underwent special staining for CMV and a portion was used for microbiological cultures and/or direct staining for pathogens. Patients underwent gastroscopy at annual intervals, colo- or rectoscopy at five years’ intervals or when clinically indicated. Chest computed tomography was performed per protocol every year and in case of acute graft dysfunction or if graft pathologies were suspected. Patients also underwent oncologic screening and neuro-psychological examination as indicated.
Infection prophylaxis, monitoring, and definitions
Infection was assumed in the case of isolation or identification of a pathogen from an otherwise sterile site in the presence of specific clinical or laboratory parameters, which led to therapeutic intervention—in most cases antibiotic treatment. Positive surveillance cultures without clinical symptoms were considered colonization. Fungal infections were judged according to the clinical presentation; invasive aspergillosis was defined as tissue invasion as opposed to Aspergillus tracheobronchitis and all cases were proven by isolation of the pathogen (37). Herpes simplex and varicella zoster virus associated disease was defined as specific lesions. CMV infection and diseases were classified according the definitions by Ljungman et al. (38). CMV infection was assumed if a specific assay was positive in the absence of clinical symptoms, CMV disease if any CMV test was positive in the presence of clinical symptoms such as neutropenia and/or fever and/or flu-like-syndrome, which could not be explained otherwise. CMV pneumonitis was suspected if X-ray or CT scan showed interstitial pneumonia and any test revealed CMV including early antigen (EA) from bronchoalveolar lavage. CMV infection was monitored by using the pp65-antigenemia assay, the Digene Hybrid Capture CMV DNA assay or CMV PCR (Amplicor Roche). Refractory CMV infection or disease was defined clinically as persistence of CMV typical symptoms and/or persistent viremia. UL97 and UL54 sequencing for resistance mutation was tested by PCR sequencing (after 2002).
Oral ganciclovir (3×1,000 mg for 3 months) was given to 48 patients. Antifungal prophylaxis was done with amphotericin B inhalations twice daily for the first 14 to 60 days according to endobronchial findings at the bronchial anastomosis. Blood-cultures (aerobic and anaerobic) were taken in case of fever >38 centigrade and reported out negative in the absence of pathogen growth after seven days. Sputum, urine, stool, aspirations and smears were taken whenever clinically indicated and as per protocol during hospitalizations.
Definition of other complications
Definitions of comorbidities followed internationally accepted guidelines by the WHO or various international societies and adapted to the specific population. A blood pressure higher than 160 systolic to 95 diastolic was defined as hypertension. Diabetes mellitus defined as fasting glucose levels of >120 mg/dL or two-hours glucose value >180 mg/dL or HbA1c levels >7 mg/dL. Renal failure was defined as glomerular filtration rate (GFR) <50 mL/min or serum creatinine >1.4 mg/dL. Osteoporosis was defined as a rarefication of bone mass (Z-score <−2). Diagnosis of GERD was based on specific symptoms and diagnosis was confirmed by esophago-gastro-duodenoscopy.
Data collection and statistical analysis
For analysis, patients’ reports were reviewed in detail for the perioperative period as well as follow up. Data were collected from clinical charts and from the hospital records. For the perioperative course, immunosuppression, body temperature, blood chemistry, all results of microbiological investigations and all applied anti-infective drugs were retrospectively collected and entered into a computerized database (EXCEL, Microsoft). All significant complications from the date of transplant until last follow up were recorded. Patients alive at last follow up were further analyzed in detail. The study was conducted according to the recommendations of the local ethics committee. Statistical analysis was carried out using SPSS. Data are reported as median with range, mean ± SD or 25%/75% quartile values. Survival was calculated using Kaplan-Meier curves. A P value of <0.05 was considered statistically significant.
Results
Patient and graft survival
Median patient survival was 978 (range, 0.5–5,276) days. A total of 39 patients (76.4%) in this series died. There was one intraoperative death due to massive pulmonary emboli and one patient died on day three post transplant due to a thrombosis of the basilary vein. Another three deaths occurred during the first post transplant month (pneumonia 2, intracerebral bleeding 1). Between one month and one year, 12 patients died (pneumonia 6, invasive aspergillosis 1, CMV disease 1, sepsis 2, erosion bleeding of a bronchial stent with Aspergillus infection 1, progressive graft failure 1). Causes of death of the remaining 22 patients who died after the first post transplant year were pneumonia (n=6), sepsis due to infected hematoma and of intestinal ischemia (n=2), invasive aspergillosis (n=2), Candida peritonitis (n=1), lung abscess (n=1), intracerebral hemorrhage (n=1), myocardial infarction (n=1) and CMV disease with chronic rejection and pancreatitis (n=1); in seven cases, the cause of death was multifactorial or unknown. A total of 21 patients (41%) in this series died from graft infection (bacterial pneumonia 8, CMV pneumonitis 7, pulmonary aspergillosis 5, non-CMV viral pneumonia 1).
Overall 1-, 5- and 10-year actuarial patient survival was 71.4%, 41.2% and 25.4%. There were three re-transplants during the study entry date (32, 357 and 1,296 days post first LuTx), all three patients died within one year after their second transplant. One patient was transplanted later on; for this patient, data were analyzed until date of re-LuTx. The median follow-up for four males and eight female recipients, who are currently alive with their first graft was 10.7 (range, 9.7–14.2) years; their median age at last follow up was 56.47 (range, 34.9–68.8) years.
Perioperative course
Prior to first LuTx, 13 patients had undergone thoracotomies including six lung volume reduction, two pleural resections, one lung resection, two open biopsies, one closure of an open duct of Botalli and one closure of a bronchial fistula. Four patients had a tracheostomy at the time of transplant. Seventeen transplants were performed using a cardiopulmonary bypass. Severe post transplant reperfusion injury of the graft was observed in 13 cases. The mean length of post-transplant hospitalization was 22 (range, 0.5–53) days. Excluding the two perioperative deaths, the overall rejection rate during first hospitalization was 35% (22 rejection episodes in 18 patients were treated). Surgical complications were observed following 22 transplants (40.7%), in two cases two complications occurred. There were five hemorrhages requiring surgical re-intervention, one splenic artery aneurysm, one colonic perforation, one perforated duodenal ulcer and one perforated gallbladder (CMV cholecystitis), one chylothorax, four cases of pneumothorax and four cases of pulmonary arterial embolism and one intraoperative air-embolism. One patient presented with a paresis of the recurrent nerve. One patient developed an abdominal aortic dissection, which most likely originated from a femoral cannula inserted during aortopulmonary bypass. Another patient underwent stenting of the pulmonary artery due to an anastomotic stenosis. There were 11 cases of bronchial anastomotic complications including dehiscence [3], stenosis [6] and others [1]. Treatment included conservative management [4], stenting [3] and surgical revision [4]. In this serie stenosis occurred mainly in the anastomotic regions (2/3), peripheral stenosis of the central airways was located in the bronchus intermedius or in the left or right lower lobe bronchi. About 60% were associated with fungal and or bacterial colonization or infection. In one case, necrosis of the bronchial anastomosis with complete breakdown led to graft failure requiring re-transplantation.
Rejection and bronchiolitis obliterans syndrome
Overall, 106 rejection episodes following 43 transplants (79.6%) were diagnosed. Of those, 56 episodes (34 patients) were diagnosed within the first year, 36 episodes in 20 patients between the second and the fifth year and 14 episodes in nine patients after the fifth year. This includes 15 cases of chronic rejection [obliterative bronchiolitis (OB)]; with six being diagnosed after the fifth year. Refractory acute rejections were defined as ongoing > A1 rejection proved histologically 3 weeks after steroid treatment of the initial rejection episode. Treatment included a second steroid bolus or additional anti-lymphocyte treatment with ATG or OKT3 at that time.
Infectious complications
A total of 399 infectious episodes were recorded. During the early post transplant period, 46 patients (85.1%) developed 146 infectious episodes (3.2 per LuTx). Between the first and fifth year, 194 infections were reported in 29 recipients (5.8 per LuTx). After the fifth year post LuTx 85 infectious episodes were diagnosed in the remaining 20 patients (4.3 per patient). All twelve long term survivors had multiple infections during follow up. Table 1 shows the time course of infectious complications and Table 2 the spectrum of isolated pathogens.

Full table
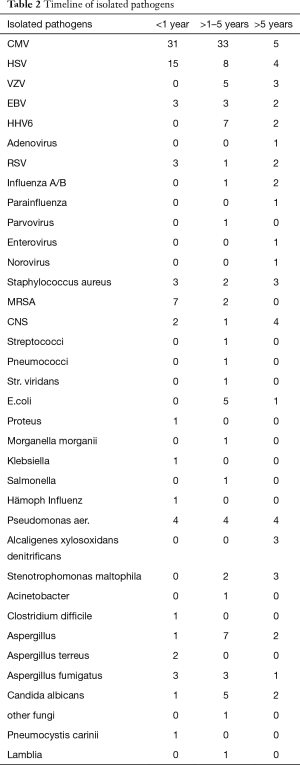
Full table
Respiratory infections
The most common infectious complications involved the respiratory tract with 180 episodes; this includes 161 episodes of graft bronchitis/pneumonia.
HSV, VZV, HHV 6, EBV
A total of 23 patients (45%) developed 27 episodes of infections caused by HSV 1 and HSV 2. Five patients developed eight episodes of VZV associated diseases (single dermatome shingles 3, generalized shingles 1 and blepharitis 1). EBV viremia was observed in seven patients with six having asymptomatic EBV reactivation. EBV associated PTLD was diagnosed in one patient and presented as nodule within the abdominal wall, which was surgically removed and revealed a diffuse large cell B-cell lymphoma. The patient well responded to surgical therapy and tapering of immunosuppression and died more than 15 years post transplant from multi organ failure. HHV 6 replication was observed in eight patients but in no case specific symptoms were seen.
CMV infection and disease
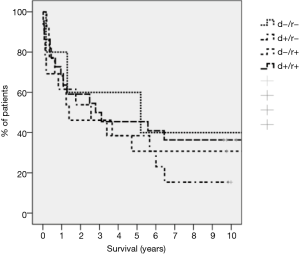
A total of 68% of recipients as opposed to 61% of donors were CMV positive. The distribution of the CMV match for this cohort was d<−/r<− (n=6, 11.1%), d+/r<− (n=12, 22.2%), d−/r+ (n=14, 25.9%), d+/r+ (n=22, 40.7%). The CMV match had no influence on long term survival (Figure 1). Thirty-one patients (57.4%) developed 72 episodes of CMV infection or disease, with 16 patients having multiple episodes. Seventeen patients developed 23 episodes of CMV infection, fourteen patients developed 27 episodes of CMV disease, thirteen patients had 20 episodes of CMV graft pneumonitis and two patients had an episode of gastrointestinal CMV disease (cholecystitis 1, gastroenteritis 1). The incidence of CMV infection/disease was highest in the mismatched population (83.3%) and lowest in sero-negative matched (d−/r−) recipients (33.3%). Of the 72 episodes, 32 developed during the first year, 35 between the second and fifth year and only five after the fifth year. About 70% of the “late” infections were recurrent infections/reactivations in patients with previous episodes and treatments of their CMV-disease. The routine CMV prophylaxis in the early patients of this serie was retrospectively inadequate and with the availability of valganciclovir, cidofovir and foscarnet and as well as early detecting tools as PCR and sequencing of clinical resistant strains improved the management recently. Two patients had CMV disease with ganciclovir resistant strains and were treated with foscarnet or cidofovir in combination with CMV immune globulin. Nine patients (18%) died directly or indirectly related to CMV disease. Five patients progressed to BOS/COP after recurrent episodes of CMV pneumonia (including the two patients with ganciclovir resistant strains) and died from graft failure commonly associated with graft pneumonia caused by other organisms. In addition, CMV disease was a contributing cause of death in one patient with bacterial sepsis, in one with rejection and pancreatitis and in two individuals with bacterial pneumonia.
Fungi
A total of 46 episodes of fungal infections were detected in 27 recipients. In the majority of cases Aspergillus spp. were isolated. Excluding the three re-transplants and the two perioperative deaths the incidence of aspergillosis was 35.4%. A total of 18 patients developed Aspergillus infection including Aspergillus tracheobronchitis (n=7) and invasive aspergillosis (n=7), chronic bronchial Aspergillus colonization (n=3) and Aspergillus fumigates associated broncho centric granulomatosis (n=1). Ten patients had recurrent episodes of aspergillosis. All cases of invasive aspergillosis were fatal. In five cases aspergillosis was the leading cause of death, in two cases invasive aspergillosis complicated CMV associated Organizing pneumonia (COP). All cases of tracheobronchitis due to Aspergillus were successfully treated. For the cases of invasive disease five of the seven patients had concomitant other infections including CMV (n=2), clostridial enterocolitis [1], MRSA pneumonia [1] and peritonitis [1]. Eight patients had Candida infections and one patient developed Pneumocystis jirovecii pneumonia whilst under TMPS routine prophylaxis and well responded to an increase in the dosage of TMPS.
Miscellaneous complications
Pulmonary embolism
There was one case of intraoperative pulmonary embolism. During follow up, pulmonary embolism was reported in thirteen patients, four out of these patients had multiple events. Six cases (11.7%) occurred within the first year (two with relapses), four (7.8%) between the second and fifth year (one with relapses) and another three (5.8%) after the fifth year; one patient developed chronic embolism. None of these events was fatal.
Pneumothorax
Eight patients presented with a pneumothorax within the first year (seven following bronchoscopy), one spontaneous pneumothorax developed between the second and fifth year. All patients were managed with chest tubes, none of these complications was fatal.
Osteoporosis
In total 28 patients developed osteoporosis. Four cases were diagnosed within the first year, 17 between the second and the fifth year and the remaining seven cases developed after the fifth post transplant year. Nine of the twelve long term survivors had developed osteoporosis. Complications of osteoporosis are displayed in Table 3.
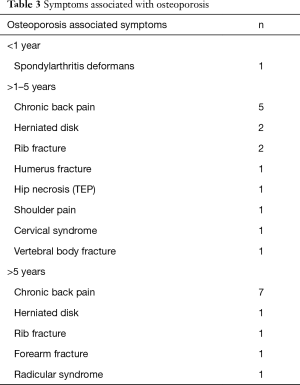
Full table
All patients received calcium and vitamin D prophylaxis in cases of reduced bone density pre-transplant. Treatment of osteoporosis included administration of bisphosphonates, calcium, vitamin D or vitamin D analoga in case of renal impairment.
Renal insufficiency
Within the first year five (15.2%) developed renal impairment and one patient (3%) renal failure requiring hemodialysis. Between the second and fifth year, 16 patients developed renal insufficiency. Thirteen suffered from impairment (creatinine >1.5 mg/dL), two from insufficiency (creatinine >2 mg/dL) and one patient had kidney-failure requiring dialysis. After the fifth year, ten patients had developed renal impairment, three insufficiencies and one patient renal failure. Of the 12 long term survivors, five had mild elevation of the serum creatinine, four had creatinine values exceeding 2 mg/dL and one patient required hemodialysis.
Diabetes mellitus
Ten patients developed diabetes mellitus: three cases were reported during the first year, two between the second and fifth year and three had new onset DM after the fifth year. Only a single patient of the 12 long term survivors has DM.
Hypertension
Hypertension was diagnosed in 23 patients. Five patients developed hypertension within the first year, 12 between second and fifth and six after the fifth year. Of the 12 patients, who are still alive, 11 (91.6%) require antihypertensive medication.
GERD
Eight patients were diagnosed with GERD; all were treated with proton pump inhibitors, no patient required fundoplication. Five cases were diagnosed between the second and the fifth year and after the fifth year. Five long term survivors suffered from GERD.
Neuro-psychiatric disorders
Nine patients developed neurological complications including three cases of transient post LuTx delirium, one stroke, one coma, two intracerebral hemorrhages, one polyneuropathy and one paresis of the recurrent nerve. One patient presented with personality disorder and poor compliance; he is alive but refuses to be followed by our transplant program. Another patient with poor compliance has developed severe alcoholism. Two patients alive at last follow up were active smokers.
Malignancies and PTLD
Ten patients developed a total of 13 malignancies (Table 4); one during the first year (PTLD), four between the second and the fifth post transplant year and five patients after the fifth year. In addition to the PTLD, there were five skin cancers (melanoma 1, basal cell cancer 1, squamous cell cancer 3 and Bowen cancer 1), recurrent pulmonary adenocarcinoma (lepidic adenocarcinoma, former bronchioloalveolar cancer) 1, glioma 1, pulmonary precancerosis 1, vulvar cancer 1. No patient died from cancer. Of the twelve long term survivors, five had developed malignancies.

Full table
Detailed analysis of twelve patients alive at last follow up
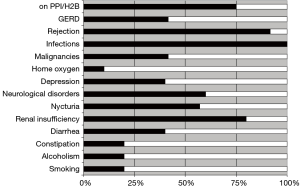
Patients were very well informed about the overall condition and well being of all other patients in this cohort. Patients were also highly knowledgeable with regard to their medication, their comorbidities and their graft function. All except two patients were highly motivated and compliant and overall satisfaction rate was very good with an awareness of the fact that survival of >10 years post LuTx is still not universally achievable. Figure 2 summarizes the overall prevalence of common complications in this cohort. Immunosuppression at last follow up included CsA (n=4), TAC (n=6), MMF (n=9), AZA (n=1); all patients were maintained on steroids. The median number of prescribed drugs was 11 (range, 6–13) per patient.
Discussion
We here in present a longitudinal cohort study of all patients undergoing LuTx between 1993 and 2000 at our center. The one-year survival in this cohort was 71%. In recent years, survival rates of 80–90% after one year were reported. Factors associated with better survival during recent years include better immunosuppressive protocols, better patient selection and new agents for therapy of perioperative complications and improved antiviral and antifungal prophylaxis and therapy. Only few studies thus far reported outcome of LuTx beyond 10 years. Of the 51 study patients, currently twelve are alive and ten participated in an assessment of their quality of life and medical condition. Rates of infection, rejection and hypertension approached 100%, other common complications include osteoporosis, DM and chronic renal failure. However, overall quality of life was reported to be good.
LuTx has become widely accepted as a life saving procedure for patients with terminal respiratory failure. In our series of 54 consecutive lung transplants using ATG or IL-2 receptor antagonist induction, CsA/TAC together with azathioprine/MMF and steroids prophylactic immunosuppression, the 1-year patient survival was 71.4%, 5-year survival was 41.2%, rejection rate was 79.6% and incidence of infectious complication was almost 100%. This retrospective analysis demonstrates that infection remains the most common complication following LuTx and infection accounted for the majority of deaths; in 20 patients, pulmonary infection was a significant factor contributing to fatal outcome. However, also a wide spectrum of other complications must be considered. This includes DM, hypertension, osteoporosis, GERD and renal impairment and malignancies. The high incidence of pulmonary embolism thus far has not been reported in lung recipients. When considering long term graft function, many factors potentially lead to graft injury and a cumulative effect must be expected. This includes donor pathologies (39), reperfusion injury, surgical complications, rejection and graft infection. Recently GERD has been reported to play a crucial role in the development of BOS. Also pulmonary embolism must be expected to deteriorate lung graft function. Finally, there is a significant proportion of patient who will start smoking again after LuTx.
During the early period of the study ATG was used for induction and replaced by IL-2 receptor antagonists. This was not associated with an increase in the rejection rate. Most patients in this series received CsA based immunosuppression. With newer protocols that include tacrolimus and m-TOR inhibitors, a significant reduction in the rejection rates was achieved together with improved short and long term survival.
Nosocomial bacterial and fungal infections, in particular pneumonia and sepsis remain significant sources of morbidity and mortality during the immediate postoperative period (40). One of the leading long term complications is graft infection with CMV and Aspergillus remaining major threats for these patients (41-45). A variety of changes in the antimicrobial prophylaxis have been made during the study period. For patients with chronic pre-transplant infections, directed prophylaxis according to pre-transplant microbiological investigations was used (46). For the first 13 transplants of this series a second generation cephalosporin and selective small bowel flora suppression was used for antimicrobial prophylaxis. This regimen was replaced by piperacillin/tazobactam and for the last cases cefepime was administered. The majority of patients in this cohort received a three months course of CMV prophylaxis with conventional ganciclovir as valganciclovir was not available. Improved outcome using valganciclovir, addition of CMV-immunglobuline and prolonged application of antivirals has been reported (47-49). Patients who undergo CMV mismatch transplantation remain at increased risk for CMV disease and may also have poorer survival (50,51). In our cohort, the CMV match had no impact on survival. Two CMV negative patients who received a graft from a CMV negative donor developed CMV infection/disease. In the first case, CMV disease developed within one-week post transplant and a false negative test in the donor or transmission with blood may be responsible. One patient developed CMV disease five years post transplant and we assume that this was a new infection acquired by natural transmission. During the observation period of this study the prophylactic regimen, the availability of antiviral drugs and the awareness of CMV disease changes dramatically as it was obvious that viral infections in lung transplant recipients were the most fatal complication (48,52).
Filamentous fungal infections accounted for significant morbidity and mortality in our patients. With the introduction of echinocandins, voriconazole and posaconazole, aspergillosis has become much better treatable and some centers also use these agents for prophylaxis. However, recently an increase in the incidence of rare fungal infections such as zygomycosis has been observed. Also, voriconazole and posaconazole interact with the metabolism of calcineurin inhibitors and toxicity due to increased levels of these agents have been observed. We have used the new antifungal agents successfully in our patients once they were available after 2003 and outcome of aspergillus infections had improved, however, the incidence of non-aspergillus-fumigatus fungi (i.e., mucormycosis, penicillium, absidia) and voriconazole-resistant molds has emerged and increased morbidity and mortality in these patients (53). Prophylaxis regimen was not changed in elective transplants before 2006 except for pre-emptive therapies in case of mold colonization with voriconazole or posaconazole.
In addition to rejection and infection, many different complications are observed in lung transplant recipients and many are due to the side effects of chronic immunosuppression. Renal insufficiency and hypertension is amongst the most commonly observed side effects but patients are also at risk to develop osteoporosis, DM and various malignancies amongst many other disorders. For future cohorts with more long term survivors, individualized immunosuppression will be necessary in order to further improve outcome and prevent some of these side effects, which certainly have a negative impact on the survival and the quality of life following LuTx. However, despite the many complications long term survivors experience, their overall satisfaction rate was very good. Compliance was good with the exception of two patients who restarted smoking and one patient who developed alcoholism.
In summary, the overall infection and rejection rate remains high and infection represents the most common cause of death in this series throughout the entire study period.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Japanese Society of Lung and Heart-Lung Transplantation. The registry report of Japanese lung transplantation--2009. Nihon Kokyuki Gakkai Zasshi 2010;48:546-9. [PubMed]
- Christie JD, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report--2008. J Heart Lung Transplant 2008;27:957-69. [Crossref] [PubMed]
- Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant 2007;26:782-95. [Crossref] [PubMed]
- Duncan MD, Wilkes DS. Transplant-related immunosuppression: a review of immunosuppression and pulmonary infections. Proc Am Thorac Soc 2005;2:449-55. [Crossref] [PubMed]
- Floreth T, Bhorade SM. Current trends in immunosuppression for lung transplantation. Semin Respir Crit Care Med 2010;31:172-8. [Crossref] [PubMed]
- McShane PJ, Garrity ER Jr. Minimization of immunosuppression after lung transplantation: current trends. Transpl Int 2009;22:90-5. [Crossref] [PubMed]
- Al-Githmi I, Batawil N, Shigemura N, et al. Bronchiolitis obliterans following lung transplantation. Eur J Cardiothorac Surg 2006;30:846-51. [Crossref] [PubMed]
- Vilchez RA, Dauber J, Kusne S. Infectious etiology of bronchiolitis obliterans: the respiratory viruses connection - myth or reality? Am J Transplant 2003;3:245-9. [Crossref] [PubMed]
- Westall GP, Michaelides A, Williams TJ, et al. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation 2003;75:2064-8. [Crossref] [PubMed]
- McCartney JG, Meyer KC. Optimizing post-transplant outcomes in lung transplantation. Expert Rev Respir Med 2008;2:183-99. [Crossref] [PubMed]
- Neuringer IP, Noone P, Cicale RK, et al. Managing complications following lung transplantation. Expert Rev Respir Med 2009;3:403-23. [Crossref] [PubMed]
- Davis CS, Shankaran V, Kovacs EJ, et al. Gastroesophageal reflux disease after lung transplantation: pathophysiology and implications for treatment. Surgery 2010;148:737-44; discussion 744-5. [Crossref] [PubMed]
- Evon DM, Burker EJ, Galanko JA, et al. Depressive symptoms and mortality in lung transplant. Clin Transplant 2010;24:E201-6. [Crossref] [PubMed]
- Alexander BD, Tapson VF. Infectious complications of lung transplantation. Transpl Infect Dis 2001;3:128-37. [Crossref] [PubMed]
- Arthurs SK, Eid AJ, Deziel PJ, et al. The impact of invasive fungal diseases on survival after lung transplantation. Clin Transplant 2010;24:341-8. [Crossref] [PubMed]
- Avery RK. Infections after lung transplantation. Semin Respir Crit Care Med 2006;27:544-51. [Crossref] [PubMed]
- Shah PD, McDyer JF. Viral infections in lung transplant recipients. Semin Respir Crit Care Med 2010;31:243-54. [Crossref] [PubMed]
- Valentine VG, Bonvillain RW, Gupta MR, et al. Infections in lung allograft recipients: ganciclovir era. J Heart Lung Transplant 2008;27:528-35. [Crossref] [PubMed]
- Valentine VG, Weill D, Gupta MR, et al. Ganciclovir for cytomegalovirus: a call for indefinite prophylaxis in lung transplantation. J Heart Lung Transplant 2008;27:875-81. [Crossref] [PubMed]
- Zamora MR. Cytomegalovirus and lung transplantation. Am J Transplant 2004;4:1219-26. [Crossref] [PubMed]
- Fischer SA. Infections complicating solid organ transplantation. Surg Clin North Am 2006;86:1127-45. v-vi. [Crossref] [PubMed]
- Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007;357:2601-14. [Crossref] [PubMed]
- Bravo C, Roldán J, Roman A, et al. Tuberculosis in lung transplant recipients. Transplantation 2005;79:59-64. [Crossref] [PubMed]
- Doan ML, Mallory GB, Kaplan SL, et al. Treatment of adenovirus pneumonia with cidofovir in pediatric lung transplant recipients. J Heart Lung Transplant 2007;26:883-9. [Crossref] [PubMed]
- Larcher C, Geltner C, Fischer H, et al. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J Heart Lung Transplant 2005;24:1891-901. [Crossref] [PubMed]
- Korzeniewska A, Dyła T, Kosacka M, et al. The most common infections of lung allografts. Pneumonol Alergol Pol 2009;77:400-6. [PubMed]
- Kaiser L, Aubert JD, Pache JC, et al. Chronic rhinoviral infection in lung transplant recipients. Am J Respir Crit Care Med 2006;174:1392-9. [Crossref] [PubMed]
- Verleden GM, Vos R, van Raemdonck D, et al. Pulmonary infection defense after lung transplantation: does airway ischemia play a role? Curr Opin Organ Transplant 2010;15:568-71. [Crossref] [PubMed]
- Bakker NA, Verschuuren EA, Erasmus ME, et al. Epstein-Barr virus-DNA load monitoring late after lung transplantation: a surrogate marker of the degree of immunosuppression and a safe guide to reduce immunosuppression. Transplantation 2007;83:433-8. [Crossref] [PubMed]
- Delgado M, Fernández R, Paradela M, et al. Development of neoplasms during lung transplantation follow-up. Transplant Proc 2008;40:3094-6. [Crossref] [PubMed]
- Knoop C, Kentos A, Remmelink M, et al. Post-transplant lymphoproliferative disorders after lung transplantation: first-line treatment with rituximab may induce complete remission. Clin Transplant 2006;20:179-87. [Crossref] [PubMed]
- Meriden Z, Bullock GC, Bagg A, et al. Posttransplantation lymphoproliferative disease involving the pituitary gland. Hum Pathol 2010;41:1641-5. [Crossref] [PubMed]
- Sathy SJ, Martinu T, Youens K, et al. Symptomatic pulmonary allograft Kaposi's sarcoma in two lung transplant recipients. Am J Transplant 2008;8:1951-6. [Crossref] [PubMed]
- Kugler C, Fischer S, Gottlieb J, et al. Health-related quality of life in two hundred-eighty lung transplant recipients. J Heart Lung Transplant 2005;24:2262-8. [Crossref] [PubMed]
- Gerbase MW, Soccal PM, Spiliopoulos A, et al. Long-term health-related quality of life and walking capacity of lung recipients with and without bronchiolitis obliterans syndrome. J Heart Lung Transplant 2008;27:898-904. [Crossref] [PubMed]
- Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant 1996;15:1-15. [PubMed]
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. [Crossref] [PubMed]
- Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002;34:1094-7. [Crossref] [PubMed]
- Hennessy SA, Hranjec T, Swenson BR, et al. Donor factors are associated with bronchiolitis obliterans syndrome after lung transplantation. Ann Thorac Surg 2010;89:1555-62. [Crossref] [PubMed]
- Husain S, McCurry K, Dauber J, et al. Nocardia infection in lung transplant recipients. J Heart Lung Transplant 2002;21:354-9. [Crossref] [PubMed]
- Iversen M, Burton CM, Vand S, et al. Aspergillus infection in lung transplant patients: incidence and prognosis. Eur J Clin Microbiol Infect Dis 2007;26:879-86. [Crossref] [PubMed]
- Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation - challenging the status quo. Clin Transplant 2007;21:149-58. [Crossref] [PubMed]
- Zamora MR, Davis RD, Leonard C, et al. Management of cytomegalovirus infection in lung transplant recipients: evidence-based recommendations. Transplantation 2005;80:157-63. [Crossref] [PubMed]
- Zuk DM, Humar A, Weinkauf JG, et al. An international survey of cytomegalovirus management practices in lung transplantation. Transplantation 2010;90:672-6. [Crossref] [PubMed]
- Solé A, Morant P, Salavert M, et al. Aspergillus infections in lung transplant recipients: risk factors and outcome. Clin Microbiol Infect 2005;11:359-65. [Crossref] [PubMed]
- Murray S, Charbeneau J, Marshall BC, et al. Impact of burkholderia infection on lung transplantation in cystic fibrosis. Am J Respir Crit Care Med 2008;178:363-71. [Crossref] [PubMed]
- Jaksch P, Zweytick B, Kerschner H, et al. Cytomegalovirus prevention in high-risk lung transplant recipients: comparison of 3- vs 12-month valganciclovir therapy. J Heart Lung Transplant 2009;28:670-5. [Crossref] [PubMed]
- Ruttmann E, Geltner C, Bucher B, et al. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation 2006;81:1415-20. [Crossref] [PubMed]
- Perreas KG, McNeil K, Charman S, et al. Extended ganciclovir prophylaxis in lung transplantation. J Heart Lung Transplant 2005;24:583-7. [Crossref] [PubMed]
- Bonatti H, Tabarelli W, Ruttmann E, et al. Impact of cytomegalovirus match on survival after cardiac and lung transplantation. Am Surg 2004;70:710-4. [PubMed]
- Palmer SM, Grinnan DC, Diane Reams B, et al. Delay of CMV infection in high-risk CMV mismatch lung transplant recipients due to prophylaxis with oral ganciclovir. Clin Transplant 2004;18:179-85. [Crossref] [PubMed]
- Bonatti H, Sifri CD, Larcher C, et al. Use of cidofovir for CMV disease refractory to ganciclovir treatment in solid organ recipients. Surgical Infection in review.
- Geltner C, Lass-Flörl C. Invasive pulmonary Aspergillosis in organ transplants - Focus on lung transplants. Respir Investig 2016;54:76-84. [Crossref] [PubMed]