Hyperglycemia in septic patients: an essential stress survival response in all, a robust marker for risk stratification in some, to be messed with in none
Since the very beginning of medicine the relation between glucose and illness has been of interest to physicians, as already Hippocrates stated: “Si quis febricitanti cibum det, convalescent quidem, robur: aegrotanti vero, morbus fit.” (That nutrition, which is beneficial in the stage of convalescence from fever, would be truly injurious during the prevalence of the disease).
More recent van Vught et al. have investigated the relation of admission hyperglycemia in patients suffering from sepsis (1). In a sub-study of a prospective observational study they found that severe hyperglycemia (>200 mg/dL) but not mild hyperglycemia (141–199 mg/dL) at admission was associated with increased 30-day mortality [HR 1.66; 95% confidence interval (CI): 1.24–2.23]. This was true for both patients with known diabetes and without diabetes, which is in contrast to previous findings by e.g., Stegenga et al. who reported an association of hyperglycemia with mortality only in patients without diabetes (2).
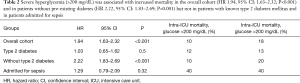
In a data set of medical critically ill patients of our own (retrospective, single-center data, 7,851 patients, 659 suffering from diabetes, 4,093 males, 522 admitted for sepsis, Table 1) severe hyperglycemia (>200 mg/dL) was associated with increased intra- intensive care unit (ICU) mortality (HR 1.94, 95% CI: 1.63–2.32; P<0.001; 10.4% vs. 18.4%) in the overall cohort and for patients without (10.1% vs. 20%; HR 2.22; 95% CI: 1.83–2.69) type 2 diabetes mellitus but not with known diabetes (12.8% vs. 13.0%; P=0.50). But interestingly, severe hyperglycemia (>200 mg/dL) was not associated with intra-ICU mortality in the sub-cohort of patients admitted to our ICU for sepsis (40% vs. 40%; P=0.926), regardless of the medical history of pre-existing diabetes (Table 2). Mortality in our sub-cohort of septic patients was with 40% intra-ICU mortality higher than in the patients suffering from sepsis investigated by van Vught et al., as overall mortality was only 27.1% in that study. We speculate that our collective was clinically sicker and in patients with septic shock even a beneficial association between hyperglycemia and mortality is in accordance to literature (3).

Full table
Full table
Of note, van Vught et al. further propose that the association of hyperglycemia and mortality is unrelated to exaggerated inflammation, endothelial cell activation and coagulation as severe hyperglycemia was associated with a decreased acute phase protein and cytokine response as well as an attenuated reduction in anticoagulant proteins such as protein C and antithrombin. This finding is surprising and new as it was thought and shown e.g., by Leonidou et al. that hyperglycemia is associated with increased pro-inflammatory cytokine production in septic patients (4).
In stress situations the body is thought to activate the central nervous system and neuroendocrine axes which release hormones such as catecholamines, glucagon and cortisol which are known to stimulate hepatic glucose production and lead to hyperglycemia (5). Stress hyperglycemia is primarily caused by hepatic gluconeogenesis and glycogenolysis rather than by peripheral insulin resistance (6). Further, hyperglycemia is thought to be at least partially physiologic and reasonable for the organism from a survival standpoint: Glucose is essential for all cells and glucose uptake is entirely dependent on a concentration gradient (though facilitated by transporters such as GLUT). In conditions like sepsis, shock or ischemia there is hypo-perfusion and reduced blood flow, therefore glucose must overcome interstitial space to reach its target, i.e., an under-perfused cell. In a situation like this a higher glucose concentration in the root, i.e., hyperglycemia, has to be considered adaptive to hypo-perfusion (7). Therefore, it is of particular interest that in the study of van Vught et al. hyperglycemia remained associated with mortality after correction for hyperlactatemia (HR 1.52; 95% CI: 1.1–2.1) in the overall cohort but not in patients without known diabetes. This could be interpreted within the meaning of a tight association of hypo-perfusion leading to hyperlactatemia and adaptive hyperglycemia in patients with a healthy glucose balance—with the relation of hypo-perfusion and hyperglycemia even excelling the association of hyperglycemia and immunological host response. Of note, the study was not optimal in regard to investigate the relation between hyperlactatemia and hyperglycemia as these values were not determined simultaneously. It certainly would be a worthy endeavor to investigate the particular relation between tissue hypo-perfusion, lactate and glucose at admission in critically ill patients.
In consistence with previous reports (8) Van Vught et al. report that preexisting diabetes did not influence 30-day mortality (30.3% vs. 26.2%; P=0.27), which we can further support by a similar finding regarding intra-ICU mortality in our own data set for both the overall cohort (11.9% vs. 12.0%, P=0.906) and septic patients (40.0% vs. 46.1%; P=0.32). This might be in contradiction to common perception as patients with diabetes mellitus are known to have an increased risk of sepsis (9), diabetes was observed to be associated with a common infectious disease (tuberculosis) as early as a thousand years ago by Avicenna (10) and diabetes is thought to be associated with an abnormal host response, impaired neutrophil chemotaxis and humoral defects (11-13). We speculate that this diminished unfavorable effect of diabetes at least in short term is most probably due to better medical intensive care treatment and effective antibiotic treatment which outplays subtler immunologic defects by diabetes.
For the clinician glucose is more than a lab value for risk stratification but a parameter which easily can be influenced by application of insulin, glucose or glucagon. Therefore, it is a question of substance how we can optimize glucose management of our patients to accomplish optimal outcome for our patients.
In 2001, van den Berghe et al. published a startling study suggesting a favorable effect of tight glucose control by intravenous insulin leading to significantly reduced mortality (14). Of note, in this single-center study mostly surgical ICU patients were investigated and in NICE-SUGAR, a large, randomized, multi-center trial demonstrated increased mortality for intensive glucose control (81 to 109 mg/dL) compared to conventional glucose control (15). Furthermore, in the studies of van den Berghe et al. a large amount, namely 87% of the calories were provided via the intravenous route, a practice we do not recommend as it was shown that the mean amount of infused glucose is independently associated with increased acute renal failure, cardiac complications and mortality (16,17). In a summary of five studies (15,18-21) comparing tight glucose control (blood glucose between 80–110 mg/dL) to control groups, an increased risk of death was reported for intensive insulin therapy and the control group showed better survival (OR 0.89; 95% CI: 0.81–0.99; P=0.04) (22). Tight glycemic control was further shown to be associated with brain energy crisis (23). Most probably the “survival benefit” for tight glycemic control reported by van den Berghe et al. was due to increased mortality in the control group because of excessive use of intravenous nutrition. For patients with septic shock even a beneficial association between hyperglycemia and mortality was reported by Tiruvoipati et al. (3). Accordingly, nowadays hyperglycemia as stress response is thought to be an evolutionary preserved adaptive and beneficial response of the organism (24).
We therefore conclude that (I) further studies investigating the relationship between hypo-perfusion and hyperglycemia are warranted; (II) hyperglycemia is a reliable marker for risk stratification of critically ill patients only in non-diabetics and patients without shock and (III) suggest a permissive and liberal management of high glucose concentrations in those patients which should focus on optimizing tissue perfusion primarily by administering fluids and ensuring proper blood pressure by means of catecholamine-therapy. As hyperglycemia should be considered to be adaptive and beneficial in critically ill septic patients we do not recommend tight glucose control and limit insulin therapy only on avoidance of fluid shifts by hyperglycemic changes of serum osmolality. As parenteral nutrition is associated with excess mortality we recommend using enteral nutrition whenever possible.
Acknowledgements
None.
Footnote
Provenance: This is an invited Commentary commissioned by the Section Editor Zhongheng Zhang (Department of Critical Care Medicine, Jinhua Municipal Central Hospital, Jinhua Hospital of Zhejiang University, Jinhua, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- van Vught LA, Wiewel MA, Klein Klouwenberg PM, et al. Admission Hyperglycemia in Critically Ill Sepsis Patients: Association With Outcome and Host Response. Crit Care Med 2016;44:1338-46. [PubMed]
- Stegenga ME, Vincent JL, Vail GM, et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med 2010;38:539-45. [PubMed]
- Tiruvoipati R, Chiezey B, Lewis D, et al. Stress hyperglycemia may not be harmful in critically ill patients with sepsis. J Crit Care 2012;27:153-8. [PubMed]
- Leonidou L, Michalaki M, Leonardou A, et al. Stress-induced hyperglycemia in patients with severe sepsis: a compromising factor for survival. Am J Med Sci 2008;336:467-71. [PubMed]
- Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab 2001;15:533-51. [PubMed]
- Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet 2009;373:1798-807. [PubMed]
- Losser MR, Damoisel C, Payen D. Bench-to-bedside review: Glucose and stress conditions in the intensive care unit. Crit Care 2010;14:231. [PubMed]
- Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care 2009;13:R18. [PubMed]
- Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 2003;26:510-3. [PubMed]
- Restrepo BI. Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances. Clin Infect Dis 2007;45:436-8. [PubMed]
- Koh GC, Peacock SJ, van der Poll T, et al. The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis 2012;31:379-88. [PubMed]
- Bannier K, Lichtenauer M, Franz M, et al. Impact of diabetes mellitus and its complications: survival and quality-of-life in critically ill patients. J Diabetes Complications 2015;29:1130-5. [PubMed]
- Jung C, Rafnsson A, Shemyakin A, et al. Different subpopulations of endothelial progenitor cells and circulating apoptotic progenitor cells in patients with vascular disease and diabetes. Int J Cardiol 2010;143:368-72. [PubMed]
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359-67. [PubMed]
- NICE-SUGAR Study Investigators, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283-97. [PubMed]
- der Voort PH, Feenstra RA, Bakker AJ, et al. Intravenous glucose intake independently related to intensive care unit and hospital mortality: an argument for glucose toxicity in critically ill patients. Clin Endocrinol (Oxf) 2006;64:141-5. [PubMed]
- Cheung NW, Napier B, Zaccaria C, et al. Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition. Diabetes Care 2005;28:2367-71. [PubMed]
- Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009;35:1738-48. [PubMed]
- Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125-39. [PubMed]
- De La Rosa Gdel C, Donado JH, Restrepo AH, et al. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care 2008;12:R120. [PubMed]
- Arabi YM, Dabbagh OC, Tamim HM, et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med 2008;36:3190-7. [PubMed]
- Marik PE. Glycemic control in critically ill patients: What to do post NICE-SUGAR? World J Gastrointest Surg 2009;1:3-5. [PubMed]
- Oddo M, Schmidt JM, Carrera E, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med 2008;36:3233-8. [PubMed]
- Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care 2013;17:305. [PubMed]


